Difference between Combination and Decomposition Reactions.
During a chemical reaction when two or more reactants form a single product then such a reaction is known as a combination reaction while a decomposition reaction is the type of reaction in which a single reactant breaks down into two or more products. An example of a combination reaction is, that calcium oxide reacts with water to form calcium hydroxide and an example of a decomposition reaction is, that calcium carbonate when heated break down into calcium oxide and carbon dioxide.
Table of content
- Combination reaction
- Examples of Combination reaction
- Exothermic reaction
- Decomposition reaction
- Endothermic reaction
- Types of decomposition reactions
- Example of decomposition reaction
You can also study
Displacement and Double displacement Reactions
Combination reaction:
A combination reaction is the type of reaction in which two or more reactants forms a single product. In simple language, we can say that when two or more substances(elements or compounds) combine to form a single product, the reaction is called a combination reaction.
Examples of Combination reactions:
1-Calcium oxide reacts vigorously with water to produce slaked lime(calcium hydroxide) releasing a large amount of heat.
CaO(s) + H2O(l) → Ca(OH)2(aq) + Heat
In this reaction calcium oxide and water combine to form a single product calcium hydroxide.
2-Burning of coal
C(s) + O2 (g)→ CO2(g)
3- Hydrogen and oxygen combine to form water
2H2(g) + O2 (g)→ 2H2O(l)
Exothermic reaction:
Click for online shopping
Future Study Point.Deal: Cloths, Laptops, Computers, Mobiles, Shoes etc
We observe that in most of the combination reactions heat is also evolved with the formation of the product. Reactions in which heat is also released along with the product formation are called exothermic chemical reactions.
Examples of exothermic chemical reactions:
1-All combustion reactions are exothermic chemical reactions because the coal, petrol, CNG, and LPG get fired at their ignition temperature and release a lot of heat.
CH4+ 2O2→ CO2+ 2H2O +Heat
2- Respiration reaction is an exothermic reaction because glucose combines with oxygen forms carbon dioxide, and water and releases heat(energy in the form of ATP).
C6H12O6 +6O2→ 6CO2+ 6H2O +ATP(Heat)
3- Vegetable oil, and compost of the remainder of animals and plants are decomposed into simpler molecules and release heat therefore these reactions are also examples of exothermic reactions.
Decomposition reaction:
A decomposition reaction is a chemical reaction in which one substance is breakdown into 2 or more substances known as a decomposition reaction.
Endothermic reaction:
It can be observed that most of the decomposed reactions are needed a certain amount of energy to break down the reactant. The type of reactions in which heat is absorbed are known as endothermic chemical reactions.
Types of decomposition reactions:
1-Thermal decomposition reaction: The type of reaction in which reactants absorb heat during the formation of the products is known as the thermal decomposition reaction.
Examples:
(i) Crystal of ferrous sulfate when heated, its green colour turns to white and then to brown, it occurs because after heating ferrous sulphate crystal(FeSO4.7H2O) loses water and anhydrous FeSO4 is formed then on further heating it is decomposed into ferric oxide with the release of sulphur dioxide and sulphur trioxide gases..
(ii)On heating calcium carbonate it decomposes into calcium oxide and carbon dioxide.
(iii)On heating lead nitrate it decomposes into lead oxide, nitrogen dioxide and oxygen.
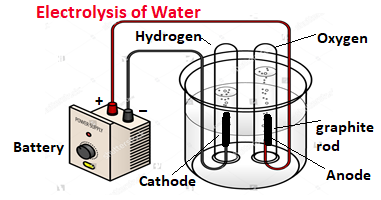
Electrolytic decomposition reaction: The type of decomposition reaction which is needed electrical energy for its decomposition is known as an electrolytic decomposition reaction. As an example, if water is passed through the process of electrolysis, it is decomposed into hydrogen and oxygen as below, hydrogen gas is separated at the cathode and oxygen is at the anode.
Photo decomposition reaction:
White silver chloride turns grey in presence of the sunlight, this is due to the decomposition of silver chloride into silver and chlorine by the light.
Class 10 Chemistry Important Notes
How to Balance the Chemical Reaction :Class 10 Chapter 1 NCERT
Important salts class 10 CBSE sceience notes
Why do calcium and magnesium float on the surface of the water?
Functioning of Soda-Acid Fire Extinguisher
What are Corrosion and Rancidity ?
Chemical properties of Acid and Bases-A note for grade 10 students
What is pH value and its importance in everyday life.
Type of Chemical Reactions with Complete detail
What are the physical and chemical properties of metals?
Extraction of metals as per the activity series
Trends in the property of element from left to right and up to down in the modern periodic table.
Ionic and covalent compounds and the difference between them
What is the difference between the soap and the detergent ?
Class 10 chemistry Viva Voce Questions and Answers for CBSE Board 2020-21
Class 9 Chemistry Important Notes
Evoporation,Vapourization and Latent heat
How does the water kept in an earthen pot become cold during summer ?
What are the factors affecting evaporation?,
What is the difference between solution,colloid and suspension?
What is the difference between element and the compound?
Determining Valency, Net Charge and Molecular Formula
Molar mass,molecular mass and mole concept
If energy is conserved then why do we need to save it for future generations?
Click the link for NCERT Solutions for classes 9,10,11 and 12 of Maths & Science
NCERT Solutions and CBSE notes for Class 9,10,11 and 12 of Maths and Science
NCERT Solutions of Class 9 Maths : from chapter 1 to 15
NCERT Solutions Class 10 Science from chapter 1 to 16
NCERT Solutions of all chapters of Maths for Class 10 from Chapters 1 to 15
NEET Syllabus and its preparation
Specific notes for the preparation of 10 class CBSE board exam for achieving excellent marks
What to do to pass in maths CBSE X th board exam ?
Tips to get success in competitive exams
NCERT Solutions for Class 10 Science
You can compensate us
Paytm number 9891436286
The money collected by us will be used for the education of poor students who leaves their study because of a lack of money.