Ionic and Covalent Compounds and the Difference between them
Ionic compounds are the compounds in which two oppositely charged ions are bounded by an electrostatic force called ionic bonds while covalent compounds are the compounds in which two or more atom similar or dissimilar shares one or more electrons in order to form an octet in the outermost orbital of the participant atoms, these shared electrons are under the impact of nuclear forces (Vander Waal forces) between the nuclei of participant atoms and shared electrons known as a covalent bond. Examples of ionic compounds are sodium chloride, potassium chloride, sodium hydroxide, calcium carbonate, etc. Examples of covalent bonds are carbon dioxide, methane, ethane, etc.
Class 10 chemistry Viva Voce Questions and Answers
Class 10 Chemistry Practical Based Questions and Answers
What is the atom, molecule, and atomicity of a substance?
How to determine Valency,net charge of an ion and Molecular formula of a substance.
What is an atom,molecule and atomicity of a substance?
Type of Chemical Reactions with Complete detail
What is pH value and its importance in everyday life.
Chemical properties of Acid and Bases-A note for grade 10 students
What are the physical and chemical properties of metals?
Difference between soaps and detergents
Class 10 chapter 2 science notes on salts
Extraction of metals as per the activity series
Ionic and covalent compounds and the difference between them
Ionic Compounds
Click for online shopping
Future Study Point.Deal: Cloths, Laptops, Computers, Mobiles, Shoes etc
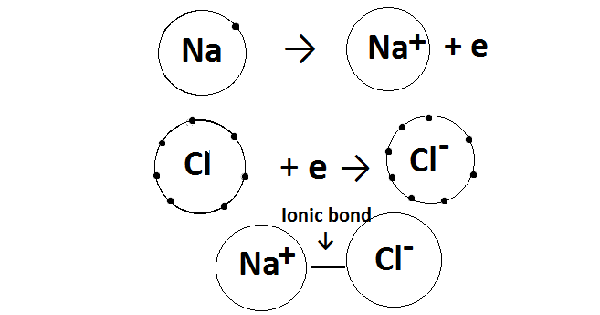
Ionic compounds are the substances that are formed after the exchange of electrons in which one atom donates one or more electrons and another atom gains those one or more electrons in order to form an octet or to get the noble gas structure.
In a response among metals and non-metals, metals commonly free electrons to complete their octet while non-metals acquire electrons to finish their octet. Metals and non-metals usually react to form ionic compounds.
The design of an ionic compound relies upon the general sizes of the cations and anions. Ionic compounds incorporate salts, oxides, hydroxides, sulfides, and most of the inorganic compounds. Ionic compounds are solid in which ions are held together by the electrostatic force between the positive and negative ions.
For instance, the sodium ions attract chloride ions and the chloride ion attract sodium ions. The outcome is a three-dimensional structure of Na+ and Cl–ions, thereby forming crystals of sodium chloride. The crystal of sodium chloride is uncharged on the grounds that the number of sodium ions is equivalent to the number of chloride ions. The powers of the force of attraction between the ions hold them in the structure.
For example reaction between aluminum and chlorine. The aluminum atom has three electrons in its outermost shell. By losing three electrons from its M shell its L shell becomes the outermost shell that has a stable octet. The nucleus of this aluminum atom still has thirteen protons but the number of electrons has decreased to ten. So, a net positive charge is developed on this aluminum atom, giving an aluminum cation Al3+.
On the other hand, the chlorine atom has seven electrons in its outermost orbital. Therefore, it needs only one electron to complete its octet. It can gain this one electron from the electrons lost by the chlorine atom to become an aluminum ion. As three electrons are lost by an aluminum atom while one chlorine atom can gain only one electron, three atoms of chlorine combine with one atom of aluminum to form aluminum chloride.
Properties of Ionic Compounds
Ionic compounds are solid and are somewhat hard because of the strong force of attraction between the positive and negative ions. These compounds are generally brittle and break into pieces when pressure is applied.
Ionic compounds have high melting and boiling points, this is because a considerable amount of energy is required to break the strong inter-ionic attraction.
Ionic compounds are generally soluble in water and insoluble in solvents such as kerosene, petrol, etc.
The conduction of electricity through a solution involves the movement of charged particles. A solution of an ionic compound in water contains ions, which move to the opposite electrode when electricity is passed through the solution. The ionic compound in the solid-state does not conduct electricity because the movement of ions in the solid-state is not possible due to their rigid structure, but ionic compounds conduct electricity in the molten state. This is possible in the molten state since the electrostatic forces of attraction between the oppositely charged ions are overcome due to the heat. Thus, the ions move freely and conduct electricity.
Covalent compounds
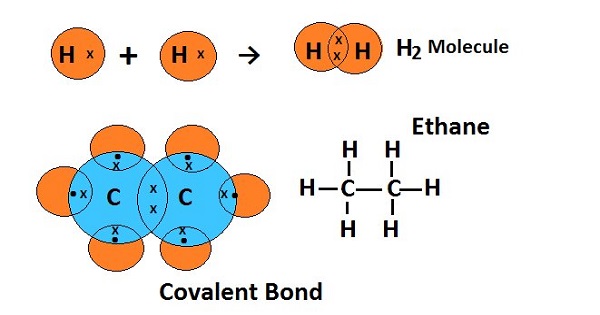
Covalent compounds are formed by the atoms of the elements which are unable to form ions due to higher ionic energy that resist to lose or gain electrons, so such atoms share one or more one electrons in order to complete octet in the outermost orbital to gain stability. Generally, covalent bonds are formed by organic compounds and molecules of non-metals, as an example.
Covalent bonding in carbon
As per the electronic configuration of carbon, it needs to gain or lose 4 electrons to become stable, which seems impossible as:
- Carbon cannot gain 4 electrons to become C4-, because it will be tough for 6 protons to hold 10 electrons and so the atom will become unstable.
- Carbon cannot lose 4 electrons to become C4+ because it would require a large amount of energy to remove out 4 electrons and also the C4+ would have only 2 electrons held by proton, which will again become unstable.
Carbon cannot gain or donate electrons, so to complete its nearest noble gas configuration, it shares electrons to form a covalent bond.
Please follow us on pintrest
Properties of covalent bond
If the normal valence of an atom is not satisfied by sharing a single electron pair between atoms, the atoms may share more than one electron pair between them. Some of the properties of covalent bonds are:
- Covalent bonding doesn’t result in the formation of new electrons. The bonds only pair them.
- They are very powerful chemical bonds that exist between atoms.
- A covalent bond normally contains the energy of about 80 Kilocalories per mole(kcal/mol)
- Covalent bonds rarely break spontaneously after it is formed.
- Covalent bonds are directional where the shells of atoms are half-filled so they showcase specific orientations relative to one another.
- Most compounds having Covalent bonds exhibit relatively low melting points and boiling points.
- Compounds with Covalent bonds usually have lower heat of vaporization.
- Covalent compounds have no free electrons hence don’t conduct electricity.
- Covalent compounds are not soluble in water.
Important Science Notes for Class 9 and 10 grade
Class 10 CBSE Science Notes
Class 10 Biology Viva Voce Questions and Answers for CBSE Board 2020-21
Class 10 Physics Viva Voce Questions and Answers
Ozone Layer and How it is Getting depleted.
Myopia, Hypermetropia and Presbyopia
Human eye structure and its function
Electrical resistance and conductance
Electric Current and Heating effect of Electric Current
What is a potential difference across an electric field ?
Why do the star twinkle but planets don’t
Light reflection, refraction, scattering, and dispersion
Food chain and food web in an ecosystem
Class 9 CBSE Science Notes
Three Laws of Motion: Class 9 CBSE
Evoporation,Vapourization and Latent heat -Class 9 CBSE notes
What is an atom,molecule and atomicity of a substance?
How to determine Valency,net charge of an ion and Molecular formula of a substance.
Thrust and Pressure : Difference
The Complete Detail of Archimedes Principal
Average Speed and Average Velocity: Differences
How to evaluate recoil velocity of gun
If energy is conserved then why do we need to save it for future generations?
Molar mass,molecular mass and mole concept
What is second law of of motion ?
What is universal law of gravitational force
NCERT Solutions of Science and Maths for Class 9,10,11 and 12
NCERT Solutions for class 9 maths
NCERT Solutions for class 9 science
CBSE Class 9-Question paper of science 2020 with solutions
CBSE Class 9-Sample paper of science
CBSE Class 9-Unsolved question paper of science 2019
NCERT Solutions for class 10 maths
CBSE Class 10-Question paper of maths 2021 with solutions
CBSE Class 10-Half yearly question paper of maths 2020 with solutions
CBSE Class 10 -Question paper of maths 2020 with solutions
CBSE Class 10-Question paper of maths 2019 with solutions
NCERT Solutions for Class 10 Science
Solutions of Class 10 Science Sample Paper and Question Papers for Term-1 and Term 2 2021-22 CBSE Board
Solution of Class 10 Science Question Paper Preboard 2021-22:Term 2 CBSE Board Exam
Solutions of Class 10 Science Sample Paper Term-1 2021-22 CBSE Board
Class 10 Science Sample Paper for Term 2 CBSE Board Exam 2021-22 with Solution
Solutions of Class 10 Science Question Paper Preboard Examination (First) 2021 -22 Class 10 Science
CBSE Class 10 – Question paper of science 2020 with solutions
CBSE class 10 -Sample paper of Science 2020
NCERT Solutions for class 11 maths
| Chapter 1-Sets | Chapter 9-Sequences and Series |
| Chapter 2- Relations and functions | Chapter 10- Straight Lines |
| Chapter 3- Trigonometry | Chapter 11-Conic Sections |
| Chapter 4-Principle of mathematical induction | Chapter 12-Introduction to three Dimensional Geometry |
| Chapter 5-Complex numbers | Chapter 13- Limits and Derivatives |
| Chapter 6- Linear Inequalities | Chapter 14-Mathematical Reasoning |
| Chapter 7- Permutations and Combinations | Chapter 15- Statistics |
| Chapter 8- Binomial Theorem | Chapter 16- Probability |
CBSE Class 11-Question paper of maths 2015
CBSE Class 11 – Second unit test of maths 2021 with solutions
NCERT solutions for class 12 maths
| Chapter 1-Relations and Functions | Chapter 9-Differential Equations |
| Chapter 2-Inverse Trigonometric Functions | Chapter 10-Vector Algebra |
| Chapter 3-Matrices | Chapter 11 – Three Dimensional Geometry |
| Chapter 4-Determinants | Chapter 12-Linear Programming |
| Chapter 5- Continuity and Differentiability | Chapter 13-Probability |
| Chapter 6- Application of Derivation | CBSE Class 12- Question paper of maths 2021 with solutions |
| Chapter 7- Integrals | |
| Chapter 8-Application of Integrals |
Class 12 Solutions of Maths Latest Sample Paper Published by CBSE for 2021-22 Term 2
Class 12 Maths Important Questions-Application of Integrals
Solutions of Class 12 Maths Question Paper of Preboard -2 Exam Term-2 CBSE Board 2021-22
Solutions of class 12 maths question paper 2021 preboard exam CBSE