Solutions of Class 10 Science Sample Paper Term-1 2021-22 CBSE Board
Solutions of Class 10 Science Sample Paper Term-1 2021-22 CBSE Board is helpful for the class 10 students in their preparation of the term 1 CBSE board exam 2021. All questions are of MCQ types ,here solutions of each question are explained by the expert of science properly which is required to every student to understand the solutions scientifically. If you go through each solution you will get bunches of scientific concepts which will help you in gaining skill to solve the science MCQ questions in Term 1 exam CBSE board .It is the most important inputs that needed to solve Term 1 CBSE Board exam.
Pdf of Solutions-Class 10 Maths Sample Paper for Term 1 2021
For extra practice you can download our e-book with solutions of class 10 maths last years 4 question papers.
Download PDF for cllass 10 solutions of last year’s maths queestion papers
The following links are also useful for class 10 maths extra preparation of the CBSE board exam of term 1.
Solutions of Class 10 Maths Question paper(Basic) 2021 CBSE Board Exam
CBSE Class 10-Half yearly question paper of maths 2020 with solutions
CBSE Class 10 -Question paper of maths 2020 with solutions
CBSE Class 10-Question paper of maths 2019 with solutions
Q1. Reema took 5 ml of Lead Nitrate solutions in a beaker and added approximately 4 ml of Potassium Iodide solution to it .What would she observe?
A.The solution turned red
B.Yellow precipitate was formed
C.White precipitate was formed
D.The reaction mixture become hot
Ans. B. Yellow precipitate was formed
Explanation:Lead Nitrate + Potassium Iodide ⇒ Lead Iodide + Potassium Nitrate
Pottassium Nitrate is soluble in water and white in colour while Lead Iodide is light yellow and partially soluble in water,so yellow precipitate of lead iodide is formed during the reaction.
Q2. Identify gas A in the following experiment
A.Nitrogen
B.Hydrogen
C.Oxygen
D.Carbon Di Oxide
Q3.
A. (i) and (iii)
B.(i) and (iv)
C.(ii) and (iii)
D.(ii) and (iv)
Ans.D (ii) and (iv)
Copper doesn’t react with HCl because ,copper lies below the hydrogen in reactivity series,so it is unable to replace hydrogen ion and doesn’t form Cucl2 and H2. Magnesium reacts fast with HCl and forms MgCl2 and H2 ,also Fe and Zn reacts with HCl on the same way pruduces their corresponding salt and H2.
Q4. Which of the following correctly represent a balanced chemical reaction.
(i) Fe (s) + H2O(g) → Fe3O4(s) +4H2(g)
(ii)3Fe(s) + 4H2O (g) →Fe3O4(s) +4H2(g)
(iii) 3Fe (s) + H2O(g) → Fe3O4(s) +4H2(g)
(iv) 3Fe (s) +4H2O(g) → Fe3O4(s) +4H2(g)
Ans.(ii) 3Fe(s) + 4H2O (g) →Fe3O4(s) +4H2(g)
Comparing LHS and RHS
In LHS and RHS the number of Iron atoms,Hydrogen atoms and Oxygen atoms are equal
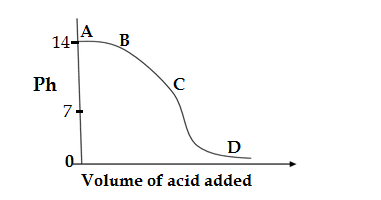
Q5.The graph given below depicts a neutrilization reaction (acid +alkali → Salt + Water). The Ph of a solution changes as we add access of acid to an alkali.
Which letter denotes the area of graph where both acid and salt are present?
A. A
B.B
C.C
D.D
Ans.D.D
In the given nuetrilization reaction ( acid +alkali → Salt + Water),according to the law of constant proportion fixed amount of the acid reacts with fixed amount of alkali(i.e base),after the formation of salt ,the excess acid added doesn’t react with alkali so forms a solution of salt and acid,so its Ph decreases ,so in the area D ,the acid and salts are present.
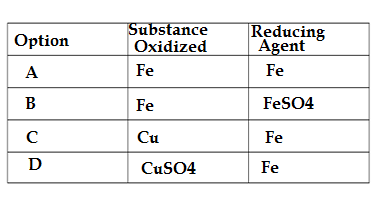
Q6.In the rection of iron with copper sulphate solution:
CuSO4 + Fe → Cu + FeSO4
Which option in the given table correctly represents the substance oxidised and the reducing agent ?
Ans. A
In the given reaction CuSO4 + Fe → Cu + FeSO4, Fe gains oxygen atom and forms FeSO4,so Fe is oxidized to FeSO4 and CuSO4 losses oxygen atom and forms Cu,so CuSO4 is reduced to Cu,here reducing agent means which enable other item to reduce(i.e loss of oxygen atom),so Fe is reducing agent.
Q7.The chemical reaction between copper and oxygen can be categrized as:
A.Displacement reaction
B.Decomposition reaction
C.Combination reaction
D.Double displacement reaction
Ans. C.Combination reaction
When two or more than two substance forms one substance then it is known as combination reaction, in the case copper reacts with oxygen forms copper oxide(Cu +O →CuO) is an example of combination reaction.
Q8.Which of the given options correctly represents the parent acid and base of calcium carbonate ?
Ans.B. Parent Acid H2CO3 and Parent Base Ca(OH)2
H2CO3 + Ca(OH)2 → CaCO3 + 2H2O
Carbonic Acid reacts with Calcium Hydroxide forms Calcium Carbonate and water.
Q9. How will you protect yourself from the heat generated while diluting a concentrated acid?
A. By adding acid to water with constant stirring
B.By adding water to acid with constant stirring
C.By adding water to acid followed by base
D.By adding base to acid with constant stirring
Ans.A. By adding acid to water with constant stirring
When we add water to acid ,first few drops of water exposed to larger amount of acid and as a result it boils the water which causes splashes of acid to our body,it occures because of the transformation of hydrogen ions(H+) to hydronium ions(H3O)+. Therefore for the safety purpose acid is added to water slowly with continue stirring the solution,heat generated during the reaction is absorbed by the larger amount of water that prevents to boil the solution and generatation of splash of acid.
Q10. Why is it important to balance a chemical equation?
A.To verify law of conservation of energy
B.To verify law of constant proportion
C.To verify law of conservation of mass
D.To verify law of conservation of momentum
Ans.C.To verify law of conservation of mass.
In a chemical reaction the mass of the reactants is always equal to the mass of the products,so for verifying the law of conservation of mass we are required to balance the chemical reaction.
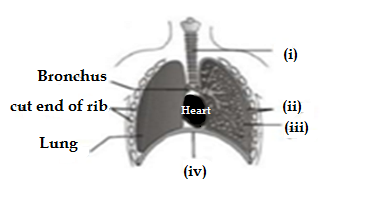
Q11. Carefully study the diagram of the human respiratory system with level A,B,C and D.Select the option which gives correct identification and main function and /or characterstics.
A.(i) Trachea :It is supported by bony rings for conducting inspired air.
B.(ii)Ribs: When we breath out,ribs are lifted.
C.(iii)Alveoli:Thin walled sac like structures for exchange of gases.
D.(iv) Diaphragm:It is pulled up when we breath in
Ans.C.(iii)Alveoli:Thin walled sac like structures for exchange of gases.
Alveoli are terminal ends of tree like strucure of the lungs which are thin walled sac like structures for exchange of carbondioxide and oxygen through the process of diffussion from blood to lungs and lungs to blood during the respiration .
Q12.Identify the option that indicates the correct engymes that is secreted in location A,B and C.
A.(i) lypase (ii) tripsin (iii) pepsin
B.(i) amylase (ii)pepsin (iii) trypsin
C.(i)tripsin (ii)amylase (iii)carboxylase
D.(i) permease (ii)carboxylase (iii) oxidase
Ans.B.(i) amylase (ii)pepsin (iii) trypsin
Amylase is present in the sliva secreted by the slivary gland that initiates the digestion of carbohydrate in the mouth.Pepsin is secreted by the walls of stomach and partially digest the protein present in our food.Trypsin is secereted by the pancreas and help to digest protein completely in the small intenstine .
Q13.Opening and closing stomatal pore depends on:
A.Atmoshpheric temperature
B.Oxygen concentration and stomata
C.Carbon dioxide concentaration around stomata
D.Water contents in the guard cell
Ans. D.Water contents in the guard cell
When water flows into the guard cells ,they swell up and the curved surface causes the stomata to open ,during the process of transpiration the guard cell shrink up and become flaccid and straight thus closing the stomata.
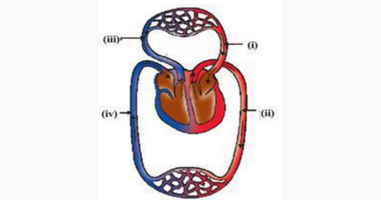
Q14.The figure given below shows the schematic plan of blood circulation in humans with levels (i) to (iv). Identify the correct label with its functions?
A.(i) Pulmonary veins-takes impure blood from body parts
B.(ii) Pulmonary artery -takes blood from lungs to heart
C.(iii) Aorta -takes blood from heart to body parts
D.(iv) Vena cava takes blood from body parts to right auricle
Ans.C.(iii) Aorta -takes blood from the heart to body parts
Aorta is the largest blood vessel which is an artery. The pulmonary vein takes blood from the lungs to the heart which opens in the left atrium. When the left artery dilates it gets blood from the pulmonary veins. When the left atrium is full of blood it contracts then the left ventricle dilates and gets blood. When the left ventricle is full of blood then it contracts and then the valve between the left ventricle and the aorta opens and blood is pumped out to all tissues of our body.
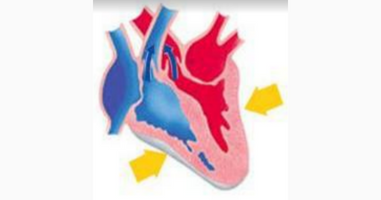
Q15.Identify the phase of circulation which is represented in the diagram of the heart given below. Arrows indicate contraction of the chambers shown.
A.Blood transferred to the right ventricle and left ventricle simultaneously.
B. Blood is transferred to the lungs for oxygenation and is pumped into various organs simultaneously.
C.Blood transferred to the right auricle and left auricle simultaneously
D.Blood is received from the lungs after oxygenation and is received from various organs of the body.
Ans.B. Blood is transferred to the lungs for oxygenation and is pumped into various organs simultaneously.
The arrow mark indicates the right ventricle and left ventricle, the right ventricle gets blood from the right auricle and then sends blood to the lungs through the pulmonary artery for oxygenation, and the left ventricle gets oxygenated blood from the left auricle and sends blood to all organs of the our body through the aorta.
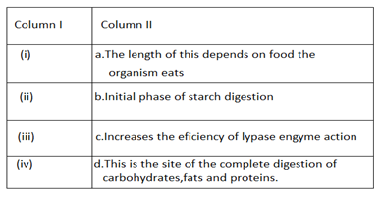
Q16.Observe the diagram of the human digestive system.
Match the labeling referred in column I and correlate with the function in colum II
A.i-a) ii-b) iii-c) iv-d)
B.i-b) ii-c) iii-d) iv-a)
C.i-b) ii-d) iii-c) iv-a)
D..i-d) ii-a) iii-b) iv-c)
Ans.B.i-b) ii-c) iii-d) iv-a)
Part(i) in the diagram is the mouth where saliva is secreted by the salivary gland mixed with food, saliva contains a specific enzyme Amylase initiates the digestion of carbohydrates in the mouth.part (ii) is the liver which secretes biles to help to emulsify fat increases the efficiency of lipase in the digestion of fat. Part (iii) is the first segment of the intestine which called the duodenum,here our food breakdown completely into simple molecule and (iv) part shows intestine, its length is different for the different organism as per the type of food they take.
Q17.Which of the following mirror is used by a dentist to examine a small cavity in a patient’s teeth?
A. Convex mirror
B.Plane mirror
C.Concave mirror
D.Any spherical mirror
Ans.C.Concave mirror
Concave mirror shows a magnified image of the object,it is that’s why dentist become capable to see the image of the mouth larger and he examine every teeth accurately.
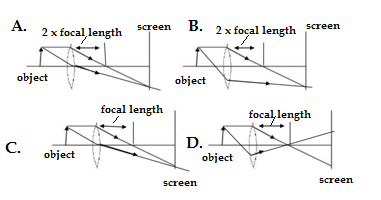
Q18.Which diagram shows image formation of an object on a screen by a converging lens?
Ans.C
The ray diagram of the image formation by a convex lense is shown as one parallel ray passes through the focal point and other ray should pass through the optical centre of the lense.
Q19.Which of the following can make a parallel beam of light when light from a point source is incident on it?
A. Concave mirror as well as a convex lense
B.Convex mirror as well as concave lense
C.Two plane mirror placed at 90° to each others
D.Concave mirror as well as concave lense
Ans.A. Concave mirror as well as a convex lense
When object(point source) is kept at the focal point of the concave mirror,it reflects the light rays parallel to the principal axis on the same way when the point source is kept at the focal point of the convex lens it refracts the light ray parallel to principal axis in contrary the convex mirror and concave lense diverges the light rays when object is positioned on the same condition.
Q20.Consider these indices of refraction: glass :1.52 air:1.0003 water:1.333. Based on the refractive indices of three materials, arrange the speed of light through them in decreasing order.
A.The speed of light in water> the speed of light in air> in the speed of light in glass
B.The speed of light in glass >the speed of light in water>the speed of light air
C.The speed of light in air >the speed of light in water>the speed of light in glass
D.The speed of light in glass >the speed of light air >the speed of light in water
Ans.C.The speed of light air >the speed of light in water>the speed of light in glass
Refractive indices of a matter= Speed of light in vacuum/Speed of light in the matter
∴Speed of light in the matter = Speed of light in vacuum/Refractive indices of the matter
Therefore it is clear that refractive indices of the matter is more ,the speed of the light in it would be decreased.
Hence order of the speeds in the given substances must be their refractive indices on their increasing order
Q21.If beam of red light and beam of violet light are incident at the same angle on the inclined surface of the prism from air medium and produce angle of refraction r and v respectively,which of the following is correct?
A.r>v
B.r>v
C.r = 1/v
D.r <v
Ans. D.r <v
Red light having larger wavelength deviates lesser than the lower wavelength violet light,so refractive angle of red beam is lesser than refractive angle of violet colour light.
Q22.
Examine the above figure and state which of the following option is correct?
[one small box in the figure is equal to 1 cm]
A.The mirror has a focal length of -6 cm and will produce an image of magnification +1
B.The mirror has a focal length of -3 cm and will produce an image of magnification -1
C.The mirror has a focal length of -3 cm and will produce an image of magnification +1
D.The mirror has a focal length of -6 cm and will produce an image of magnification -1
Ans.B.The mirror has a focal length of -3 cm and will produce an image of magnification -1
Focal length of the mirror is PF (which consist of 3 boxes) = 3×-1 = -3 cm
Since the image formed by the concave mirror is inverted ,so the magnification should be negative.B. shows both are negatives .
Q23.
The angle of incidence from air to glass at the point O on the hemispherical glass slab is
A.45°
B.0°
C.90°
D.180°
Ans.B.0°
The principal axis is the tangent at the point O and normal is along the incident ray ,the angle of incident at point O of the hemispherical glass is the angle between normal and incident ray ,since normal and incident ray coincide to each other,so angle of incident is 0°.
Q24.A prism ABC (with BC as base) placed in different orientations. A narrow beam of white light is incident on the prism as shown in below figure.In which of the following diagrams,after despersion ,the third colour from the top of the spectrum corresponds to the colour of the sky?
A.(i)
B.(ii)
C.(iii)
D.(iv)
Ans. B.(ii)
When the prism is placed BC as a base and vertex A at the top then the spectrum of seven colours from the top is Red, Orange, Yellow, Green, Blue, Indigo, Violet and when it is inverted A is below the base BC then the spectrum of colours is reversed(Violet, Indigo, Blue, Green, Yellow, Orange, Red), therefore from the top third colour corresponds to blue colour (the colour of sky).
Section B
Section B consists of 24 questions (Sl No 25 to 48) .Attempt any 20 questions from this section.The first 20 attempted questions would be evaluated.
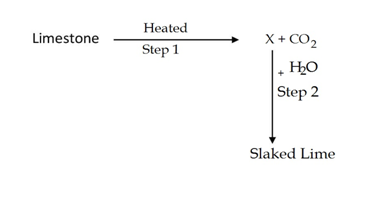
Identify the correct option from the given table which represents the type of reactions in step 1 and step 2.
Ans. C
In the given reaction when lime stone(CaCO3) is heated,it is converted into CaO, so x is Cao(Calcium oxide or quick lime),the reaction of CaO and H2O gives Calcium Hydroxide(Slaked Lime).
Since in the first reaction heat is absorbed ,therefore it is endothermic reaction while in the second reaction heat is released,therefore it is exothermic reaction. The reactions are as follows.
Q26.In which year is concentration of hydrogen ion the highest
A.2002
B.2008
C.2011
D.2005
Ans. B.2008
If PH value is high the the concentration of (H+) ions are low and if PH value is low the concentration of (H+) ions is high. Therefore the lowest value of PH in the year 2008 shows that there were highest concentration of (H+) ions in the rain as compared with other years.
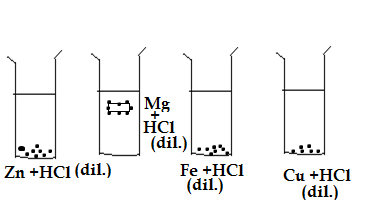
Q27.The diagram shows the reaction between metal and dil.acid.
What is the reason for different behaviour of Mg in test tube B ?
A.Mg is lighter element than dil.HCl.
B.Mg reacts with dil.HCl to produce H2 gas which helps in floating.
C.Mg reacts with dil.HCl to produce N2 gas which helps in floating.
D.Mg reacts with dil.HCl to produce CO2 gas which helps in floating.
Ans.B.Mg reacts with dil.HCl to produce H2 gas which helps in floating.
2Mg + 2HCl(dil.) → 2MgCl +H2
H2 gas is the lighest gas ,the formation of this gas around the magnesium molecules lift the molecules of Mg on the surface of water.
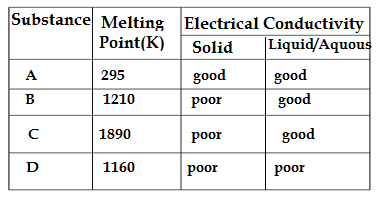
Q28.The table shown below gives information about four substances A,B,C and D.
Identify ionic compounds from the above given compounds.
A.A,B
B.B,C
C.A,B,D
D.A,C,B
Ans.B.B,C
Ionic compounds have high melting point.
Ionic compounds have poor conductivity at solid state,in liquid state or in their aquaous solutions ionic compounds are ionized ,so in liquid and aquous state they have good electrical conductivity.
Q29.Vinay observed that the stain of curry on a white shirt becomes reddish-brown when shoap is scrubbed on it ,but it turns yellow again when the shirt is washed with plenty of water .What might be the reason for his observation?
i.Soap is acidic in nature
ii.Soap is basic in nature
iii.Turmeric is a natural indicator which gives reddish tinge in bases
iv.Turmeric is a natural indicator which gives reddish tinge in acids
A. i and ii
B.ii and iii
C.i and iv
D.ii and iv
Ans. D.ii and iv
The curry contains the tomato and tomato has acids like citric acids,oxalic acids etc, also turmeric being an essential part of the curry in spices
When soap is scrubbed then the spot on the white shirt (i.e acidic in nature) , spot turns into redish brown since turmeric gives reddish colour in basic medium. When soap is rinsed with water the base(Shoap) is removed then the spot turns yellowish because turmeric gives yellow colour in acidic and neutral medium.
Q30.In which of the following steps would the bulb glow ?
A.i and ii
B.i and iv
C.ii,iii and iv
D.i,ii and iv
Ans. B.i and iv
In i step Dil.HCl releases H(+) and Cl(-) ions ,therefore electricity conducts in the solutions and bulb will glow.In iv step Lime water Ca(OH)2 gives Ca(+) and OH(-) ions,so electricity will also conduct in the solutions which results to glow the bulb.
Sugar solution and alcohol both are covalent compounds,hence there is strong C-H bonds in their molecule ,so these compounds are unable to release ions to conduct electricity.
Question no 31 to 35 consists of two statements -Assertion A and Reason R.Answer these questions selecting the appropriate options given below.
A. Bothe A and R are true and R is the correct explanation of A
B.Bothe A and B are true and R is not the correct explanation of A
C.A is true but R is false
D.A is false but R is true
Assertion:Fresh milk in which baking soda is added,takes a longer time to set as curd.
Reason:Baking soda decreases the pH value of fresh milk to below 6
Ans.C. A is true but R is false
Milk contains lactic acid ,for removing the flavour of lactic acid milk men add baking soda in it ,baking soda being as a base neuralizes the lactic acid. Since such a milk is alkaline(basic) in nature so when acid is added to it for preparing the curd it tekes a longer time in turning milk to curd.
Baking soda increases the pH value of the milk
Q32.Assertion: Decomposition of vegetable matter into compost is an endothermic reaction
Reason: Decomposition reaction involves breakdown of single reactant into simple products.
Ans. Both A and R are true and R is the correct explanation of A
The vegetable matter is decomposed by microbes which requires energy to breakdown the bonds in the matter,in turn heat energy is released so this reaction is exothermic reaction.
Q33.Assertion:Resins and gums are stored in old xylem tissue in plants
Reason: Resins and gums facilitate the transport of water molecules.
Ans.A is true but R is false
Resins and gums are excreted by the plants as a waste products which are stored in dead tissues of the plants(old xylem tissues),resins and gums doesn’t facilitate transport of water molecules.
Q34.Assertion: Sky appears blue in the day time
Reason:White light is composed of seven colours
Ans.B. Both A and R are true and R is not the correct explanation of A
Sky appears blue because ,blue colour being of small wavelength scattered by the small gas molecules of the atmoshphere.
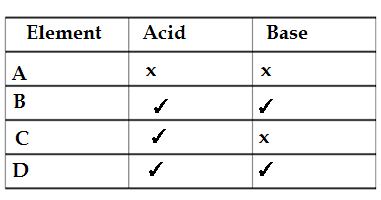
Q35.The table given below shows the reaction of few elemnents with acids and bases to evolve hydrogen gases.
Which of these elements form amphoteric oxide ?
A. A and D
B. B and D
C.A and C
D.B and C
Ans.B.B and D
Amphoteric oxide are the oxides which behaves as a acid as well as a base since these oxides neutrilize acid and base.B and D reacts with base as well as acid so B and forms amphoteric oxide .
Q36.In which of the following groups of organisms,blood flows through the heart only once during one cycle of passage through the body.
A.Rabbit,Parrot,Turtle
B.Frog,Crocodile,Pigeon
C.Whale,Labeo,Panguine
D.Shark,dog fish,sting ray
The heart of the fishes has two chambers one auricle and one ventricle and has single closed circulatory system,so blood passes through the heart only once in single cycle,means blood vessels to auricle,auricle to ventricle and venrticle to blood vessels,blood passes through the gilles exchange O2 and CO2 and again distributed to body parts and returned to the heart
You can compensate us by donating any amount of money for our survival
Our Paytm No 9891436286
NCERT Solutions of Science and Maths for Class 9,10,11 and 12
NCERT Solutions for class 9 maths
NCERT Solutions for class 9 science
CBSE Class 9-Question paper of science 2020 with solutions
CBSE Class 9-Sample paper of science
CBSE Class 9-Unsolved question paper of science 2019
NCERT Solutions for class 10 maths
CBSE Class 10-Question paper of maths 2021 with solutions
CBSE Class 10-Half yearly question paper of maths 2020 with solutions
CBSE Class 10 -Question paper of maths 2020 with solutions
CBSE Class 10-Question paper of maths 2019 with solutions
NCERT solutions of class 10 science
Solutions of class 10 last years Science question papers
CBSE Class 10 – Question paper of science 2020 with solutions
CBSE class 10 -Latest sample paper of science
NCERT solutions of class 11 maths
| Chapter 1-Sets | Chapter 9-Sequences and Series |
| Chapter 2- Relations and functions | Chapter 10- Straight Lines |
| Chapter 3- Trigonometry | Chapter 11-Conic Sections |
| Chapter 4-Principle of mathematical induction | Chapter 12-Introduction to three Dimensional Geometry |
| Chapter 5-Complex numbers | Chapter 13- Limits and Derivatives |
| Chapter 6- Linear Inequalities | Chapter 14-Mathematical Reasoning |
| Chapter 7- Permutations and Combinations | Chapter 15- Statistics |
| Chapter 8- Binomial Theorem | Chapter 16- Probability |
CBSE Class 11-Question paper of maths 2015
CBSE Class 11 – Second unit test of maths 2021 with solutions
NCERT solutions of class 12 maths
| Chapter 1-Relations and Functions | Chapter 9-Differential Equations |
| Chapter 2-Inverse Trigonometric Functions | Chapter 10-Vector Algebra |
| Chapter 3-Matrices | Chapter 11 – Three Dimensional Geometry |
| Chapter 4-Determinants | Chapter 12-Linear Programming |
| Chapter 5- Continuity and Differentiability | Chapter 13-Probability |
| Chapter 6- Application of Derivation | CBSE Class 12- Question paper of maths 2021 with solutions |
| Chapter 7- Integrals | |
| Chapter 8-Application of Integrals |