Class 10 Science Sample Paper for Term 2 CBSE Board Exam 2021-22 with Solution
Class 10 Science Sample Paper for Term 2 CBSE Board Exam 2021-22 with Solution are created here by an expert of science in order to help class 10 science students to boost their preparation for class 10 science paper in CBSE Term 2 Exam.
The feature of Class 10 Science Sample Paper for Term 2 CBSE Board Exam 2021-22 are the following.
(i)All questions are compulsory.
( ii) The question paper has three sections and 15 questions. All questions are compulsory.
(iii) Section–A has 7 questions of 2 marks each; Section–B has 6 questions of 3 marks each; and Section–C has 2 case based questions of 4 marks each.
(iv) Internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
Solution of Class 10 Maths(Basic) Sample Paper Published by CBSE for Term 2, 2022
Class 10 Science Notes for Term 2
Download PDF-Solutions of Basic and Standard Maths Sample Paper published by CBSE for Term 2 CBSE Board exam 2021-22
Solutions-Class 10 Maths(Basic) sample paper Term 2 published by CBSE-2022 Board
Solutions-Class 10 Maths(Standard) sample paper Term 2 published by CBSE-2022 Board
Class 10 Science Sample Paper for Term 2 CBSE Board Exam 2021-22 with Solution
Click for online shopping
Future Study Point.Deal: Cloths, Laptops, Computers, Mobiles, Shoes etc
SECTION-A
Q1. The table shows the electronic structures of four elements.
| Element | Electronic Structure |
| P | 2, 6 |
| Q | 2, 8, 1 |
| R | 2, 8, 7 |
| S | 2, 8, 8 |
(a) Identify which element (s) will form covalent bonds with carbon.
Ans.(a)The atomic no. of carbon is =6
Therefore its electronic structure is =2,4
The carbon atom is required 4 electrons to fulfill its outermost orbit
P requires 2 electrons to fulfill its outermost orbit, carbon requires 4 electrons, so total valence electrons are 6, therefore when P reacts with C it can form covalent bonds with two atoms by sharing their 2-2 electrons.
Q can easily form a positive ion by donating its 1 valance electron so it can form ionic bond instead of a covalent bond with carbon.
R can easily form a negative ion by accepting 1 electron so it also can form an ionic bond but its 4 atoms can combine with one atom of carbon by the formation of a covalent bond.
S is the inert gas, so it can’t form covalent and ionic bonds.
Hence P and R can form covalent bonds with carbon atom.
(b) “Carbon reacts with an element in the above table to form several compounds.” Give suitable reason.
Ans. The electronic structure of the element P (2,6) shows that it is oxygen that has an affinity to combine with a carbon atom, two oxygen atoms combined with 1 carbon atom to form CO2 by sharing 2-2 electrons which result in a double bond O to C and C to O and one oxygen atom can also combine with one carbon atom to form CO by sharing its 4 electrons resulting in a triple bond between C and O, all carbonic compounds react with oxygen freeing these two gases.
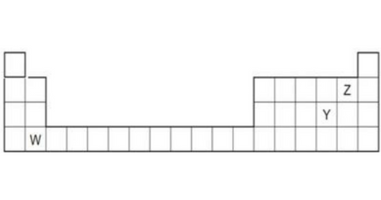
Q2. The diagram below shows part of the periodic table.
(a) Which elements would react together to form covalent compounds?
The elements which gain electrons in the formation of the compounds are capable to form a covalent compound in other words the non-metals that react together can form covalent compounds.Since y and z both are non-metals therefore both of them react together to form covalent compounds.
(b) Between the two elements W and Z, which will have a bigger atomic radius? Why?
Ans. The element W lies in the second group and Z lies in 17 th group, the atomic radius of the element decreases from left to right because of the increasing amount of the positive charge at the nucleus. Since electrons are negatively charged, the clouds of electrons constricted from left to right in the periodic table, hence the atomic radius of W is bigger than Z.
Q3.a.Trace the path a male gamete takes to fertilize a female gamete after being released from the penis.
Ans. The eggs are stored in two ovaries of the women, during ovulation, the women’s eggs travel through the fallopian tube leading to the uterus. The sperm of men enter through the vagina and then through the cervix enter to the uterus wherefrom it travels through the fallopian tube and fertilizes the egg, then the fertilized egg is further transported to the uterus and implants on the wall of the uterus and a zygote is formed.
b.State the number of sets of chromosomes present in a zygote.
Ans. The zygote is formed as a result of the fusion of the eggs and sperm, it is a diploid of two sets of chromosomes one is paternal and the other is maternal from male and female.
Q4.Rajesh observed a patch of greenish-black powdery mass on a stale piece of bread.
a. Name the organism responsible for this and its specific mode of asexual reproduction.
Ans. Patch of greenish-black powdery mass on a stale piece of bread is due to the generation of fungi (rizopous), which settle down as per the proper atmosphere(humidity and wet surface). The specific mode of reproduction is spore formation which is one of the types of assexual reproduction,in this way sporangia at the tip of the plant when matured burst into spores which are minute cells , these cells are transported through the air.
b. Name its vegetative and reproductive parts.
Ans. The thread-like structure filament and root-like structure hyphae are the vegetative part and the spherical structure sporangia that contain thin cells spores are the reproductive parts.
Q5.Mustard was growing in two fields- A and B. While Field A produced brown coloured seeds, field B produced yellow coloured seeds.
It was observed that in field A, the offsprings showed only the parental trait for consecutive generations, whereas in field B, majority of the offsprings showed a variation in the progeny.
What are the probable reasons for these?
Ans. Field A produced brown coloured seeds,it can be observed that offsprings is showed parental trait in the consecutive generation that is the result of self-pollination in which next generation is similar to the parent’s field B produced yellow coloured seeds which is the result of variation occurred in the progeny that is due to the cross-pollination since two plants of the same species are involved.
OR
In an asexually reproducing species, if a trait X exists in 5% of a population and trait Y exists in 70% of the same population, which of the two trait is likely to have arisen earlier? Give reason.
Ans. In an asexually reproducing species, identical copies of DNA are produced, so in asexual reproduction, the traits vary due to the environmental factor in a large amount of time. The trait X exists in 5% of a population and trait Y exists in 70% of the same population, since trait Y represents a larger population, it will replicate over more generations compared to trait X therefore the trait Y would have arisen early.
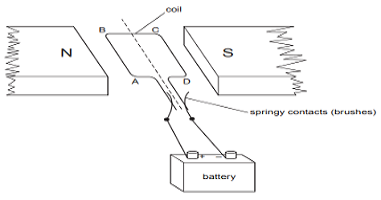
Q6.A simple motor is made in a school laboratory. A coil of wire is mounted on an axle between the poles of a horseshoe magnet, as illustrated.
In the example above, coil ABCD is horizontal and the battery is connected as shown.
a. For this position, state the direction of the force on the arm AB.
Ans. When the circuit is switched on the current is flowed from A to B, which shows the direction of the magnetic field is perpendicular to the direction of the current. According to Flaming’s left-hand rule if we stretch three fingers of the left-hand perpendicular to one another in such a way that the index fingers show the direction of the magnetic field and the middle finger shows the direction of the current then the thumb will show the direction of the force.The direction of force on AB is downwards since if we align middle finger along A to B then thumb will be downwards which shows the direction of the force.
b. Why does the current in the arm BC not contribute to the turning force on the coil?
Ans.The current in the arm BC does not contribute to the turning force on the coil because the direction of the current is from B to C along the direction of the magnetic field.
OR
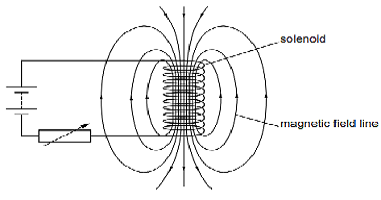
A circuit contains a battery, a variable resistor, and a solenoid. The figure below shows the magnetic field pattern produced by the current in the solenoid.
a.State how the magnetic field pattern indicates regions where the magnetic field is stronger.
Ans.The magnetic field lines shows the strength of magnetic field ,the magnetic field lines crowded at the ends of the solenoid because these field lines originates from the north poles and merge into the south pole,therefore magnetic field at both ends are stronger.The magnetic lines per unit area shows the strength of the magnetic field lines.
b.What happens to the magnetic field when the current in the circuit is reversed?
Ans.When the current in the circuit is reversed the direction of the magnetic field is also reversed.
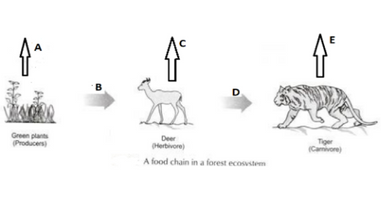
Q7.DDT was sprayed in a lake to regulate the breeding of mosquitoes. How would it affect the trophic levels in the following food chain associated with a lake? Justify your answer.
Ans.DDT is a non-biodegradable substance when it is sprayed in a lake is first consumed by the plankton, the first trophic level then plankton is eaten by the small fish, the second trophic level, small fish is consumed by the large fish, the third trophic level in the aquatic environment then large fish is eaten by the halk which lies at the fourth trophic level of the given food chain.
Non-biodegradable pesticide accumulates progressively at each trophic level, this phenomenon is known as biological magnification so a higher trophic level accumulates the largest amount of the DDT, here it is Hawk which will have the highest level of the pesticide.
OR
In the following food chain, vertical arrows indicate the energy lost to the environment and horizontal arrows indicate energy transferred to the next trophic level. Which one of the three vertical arrows (A, C and E) and which one of the two horizontal arrows (B and D) will represent more energy transfer? Give reason for your answer
Ans. In a food chain, 10 % of the energy out of the total energy driven from food is transferred to the next trophic level and rest 90% is transferred to the environment.As the trophic level increases the amount of the transferred energy is decreases.
In A,C and E, maximum energy is lost to the environment by A
In B and E , maximum energy is transferred to next trophic level by B.
SECTION B
Q8.Choose an element from period 3 of the modern periodic table that matches the description given below in each instance. Give reason for your choice.
a. It has a similar structure to diamond.
Ans. Period 3 of the modern periodic table contains the elements Na, Mg, Al, Si, P, S, Cl, and Ar
Si lies in 14 groups and has 4 valence electrons that resemble C,Si also forms a covalent bond and has catenation property just like C, therefore every atom is surrounded by 4 Si atoms forming the shape of the tetrahedron as in the diamond.
b. It has the same valency as Lithium.
Ans. The atomic number of Lithium is 3, therefore its electronic structure is 2,1 implies that its valency is 1, the same valency is of Na whose atomic no. is 11 and the electronic structure is 2,8,1.
c. It has variable valency and is a member of the Oxygen family (group 16).
Ans. The oxygen and sulphur lies on the 16 groups and has the same no. of electrons in their outermost orbit 6, therefore valancey of both elements is (-2), but the valency of sulphur varies since it forms SO2 and SO3 in which sulphur shows the valency of 4 and 6 respectively.
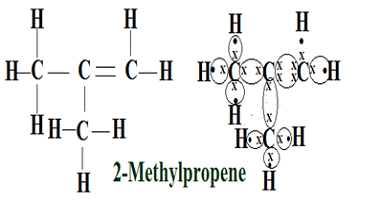
Q9.a.How many isomers are possible for the compound with the molecular formula C4H8? Draw the electron dot structure of branched-chain isomer.
Ans. The given molecular formula C4H8 is of butene,it has 4 isomers because of the double bond between two carbon atoms compared to its corresponding member butane which has two isomers only.
If a double bond is located between the first and second carbon atom then it forms 1-butene, if the double bond is located between the second and third carbon atom then it forms 2-butene but along these structural isomers it also shows one more structural and geometrical isomerism,if two methyl group(CH3) are in the same side of the double bond then it forms cis.2-butene and if they are in opposite side then it forms trans.2-butene, therefore there are total of 4 isomers of butene.
The electron dot structure of branched-chain isomer(2-Methylpropene) is
b.How will you prove that C4H8 and C5H10 are homologues?
Ans. C4H8 and C5H10 are the members of a homologues series alkene because they are differed by CH2 unit,and similar molecular formula CnH2n fo and have same chemical properties as an example both of them give addition reaction.
OR
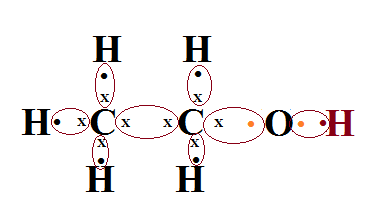
A carbon compound ‘A’ having melting point 156K and boiling point 351K, with molecular formula C2H6O is soluble in water in all proportions.
a.Identify ‘A’ and draw its electron dot structure.
Ans.The molecular formula C2H6O is can be rewritten as C2H5OH which is showing it is ethenol,having melting point 156K and boiling point 351K,therefore the given carbon compound ‘A’ is ethenol which is soluble in water in all proportions.
b.Give the molecular formulae of any two homologues of ‘A’.
Ans. The homologues of ‘A’ ehenol(C2H6O)are propanolC3H7OH (C3H7O )and butanolC4H7OH (C4H8O).
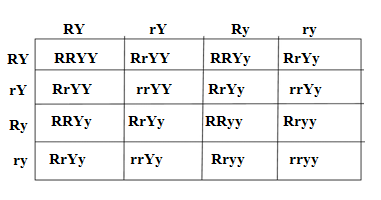
Q10.Two pea plants – one with round yellow seeds (RRYY) and another with wrinkled green (rryy) seeds produce F1 progeny that have round, yellow (RrYy) seeds.
When F1 plants are self-pollinated, which new combination of characters is expected in F2 progeny? How many seeds with these new combinations of characters will be produced when a total 160 seeds are produced in F2 generation? Explain with reason.
Ans.As per the Mendal’s law of dihybrid inheritence and law of independence inheritence two pea plants of the contrast traits – one with round yellow seeds (RRYY) and another with wrinkled green (rryy) seeds produce F1 progeny that have round, yellow (RrYy) seeds,in which round shape and yellow colour are the dominant traits.
If two plants of first generation (RrYy) are self polinated then in F2 generation different combination of shape and colour are obtained in the ratio of 9 : 3 : 3 : 1
The possible combination of the gamets RY,rY, Ry and ry
The combinations of traits of seeds in F2 generations are produced as following
The ratio of round yellow,round green,wrinkled yellow and wrinkled green seeds are 9:3:3:1
Total seeds produced in F1 generation =160
Round yellow seeds =(9/16)×160 =90
Round green seeds =(3/16)×160 =30
Wrinkled yellow seeds =(3/16)×160 =30
Wrinkled green seeds =(1/16)×160 =10
Q11.a.It would cost a man Rs. 3.50 to buy 1.0 kW h of electrical energy from the Main Electricity Board. His generator has a maximum power of 2.0 kW. The generator produces energy at this maximum power for 3 hours. Calculate how much it would cost to buy the same amount of energy from the Main Electricity Board.(1 Mark)
Ans.The cost of one unit(1 kWh) = Rs. 3.50
The power(P) of the generator is = 2 kW
The energy consumed by the generator is
E = Pt,where t is time i.e 3h,P=2 kW and E is energy produced by generator
E = 2kW×3h = 6kW h
The cost of electrical energy
=E×cost of one unit(1 kWh)
=6kW h × Rs. 3.50 =Rs21
Hence Rs 21 is the cost to buy the same amount of energy from the Main Electricity Board
b.A student boils water in an electric kettle for 20 minutes. Using the same mains supply he wants to reduce the boiling time of water. To do so should he increase or decrease the length of the heating element? Justify your answer.(2 Marks)
Ans. The time taken by electric kettle to boil water is 20 minutes
For reducing the boiling time of water the resistance of the heating element in the electric kettle should be increased as we have the relation
H =i²Rt, where H is heating energy,i is an electric current which is constant,R is resistance of heating element,t is the time taken to boil the water
So, we have
t = H/i²R
For reducing t, R should be increased
Since resistance is proportional to the length of the conductor(heating element )
R ∝ L
Therefore he should increase the length of the heating element
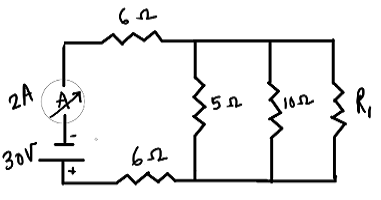
Q12.
In the above circuit, if the current reading in ammeter A is 2A, what would be the value of R1?
Ans. The resistances are 6 Ω, 5 Ω,6Ω and 16 Ω are arranged in such a way
Voltage,V =30V, current, I = 2A ,R1
The resistors 5Ω and 10 Ω and R1 are connected parallel to the battery of 30V
Their equivalent resistance is
1/Rp(eq)=1/5 +1/10+1/R1 =(2R1 +R1 +10)/10R1=(3R1 +10)/10R1
Rp(eq) =10R1/(3R1 +10)
According to Ohm’s law
V =IR
Where I=2A, V =30 V
R(total) =V/I =30/2 = 15 Ω
The resistence 6Ω,6Ω and Rp(eq) are in series
R(total) =6Ω+6Ω +Rp(eq)=12Ω +10R1/(3R1 +10)
12 +10R1/(3R1 +10)=15
36R1 +120 +10R1= 45R1+150
36R1 +10R1 -45R1 =150-120 =30
46R1 -45R1 =30
R1 =30 Ω
OR
Calculate the total resistance of the circuit and find the total current in the circuit.
Ans.6Ω and 4Ω are in series, their equivalent resistance is (6+4)Ω =10 Ω
Rp – equivalent parallel resistance of 10Ω and 10Ω
1/Rp = 1/10 +1/10 = (1 +1)/10 =2/10 =1/5
Rp =(7+5 )Ω=12Ω
R(total) =12Ω
Total current =voltage/total resistence =24V/12Ω =2 A
Q13.Gas A, found in the upper layers of the atmosphere, is a deadly poison but is essential for all living beings. The amount of this gas started declining sharply in the 1980s.
a. Identify Gas A. How is it formed at higher levels of the atmosphere?
b. Why is it essential for all living beings? State the cause for the depletion of this gas.
Ans.a.Ozone gas
Ultraviolet rays in the upper layers of the atmosphere split up oxygen molecule
O2→O +O
Oxygen atom is very reactive,it combines with oxygen molecule and forms molecule of ozone ,it is the reason that upper layer of the atmosphere is made of ozone known as ozonosphere.The
O2+O →O3
b.Ozone is a poisonous gas but its layer prevents all living organisms from the effect of ultraviolet rays, ultraviolet rays may cause skin cancer to all living organisms.The ozonosphere started declining sharply in the 1980s because of the release of the gas chlorofluorocarbon used in AC, refrigerators and sprays.
For answering this question study the following note
Ozone Layer and How it is Getting deplteted.
CASE STUDY
SECTION – C This section has 02 case-based questions (14 and 15). Each case is followed by 03 sub-questions (a, b and c). Parts a and b are compulsory. However, an internal choice has been provided in part c.
Sahil performed an experiment to study the inheritance pattern of genes. He crossed tall pea plants (TT) with short pea plants (tt) and obtained all tall plants in the F1 generation.
Q14.a.What will be the set of genes present in the F1 generation? (1 Mark)
Ans. The traits of tallness of the plant is represented by (TT) and the traits of dwarfness of the plant is represented by (tt)
He obtained all tall plants in the F1 generation
The cross of TT and tt yields the plants have the set of genes
TT ×tt =Tt,Tt,Tt,Tt
Hence the set of genes present in the F1 generation is Tt.
b.Give a reason why only tall plants are observed in F1 progeny. (1 Mark)
Ans. According to Mendel’s law of monohybrid inheritance and the law of segregation, if single pair of contrasting traits were cross-bred by self-pollination in the F1 generation, all the plants with the traits Tt are produced in which tallness of the plants is the dominant trait and shortness of the plants is a recessive trait.
c.When F1 plants were self-pollinated, a total of 800 plants were produced. How many of these would be tall, medium height or short plants? Give the genotype of F 2 generation. (2 Marks)
Ans. When two plants of F1 generation are cross pollinated then plants produced in F2 generation are
Tt ×Tt ⇒ TT,Tt,Tt,tt
When two plants of F1 generation are cross pollinated then plants pruduced in F2 generation are with the traits,then three plants can be seen with tallness and one plant is seen dwarf
Tt ×Tt→TT,Tt,Tt,tt ,these set of genes in F2 generation is the genotype of F2 generation
Three tall plants TT,Tt,Tt and one dwarf plant
The ratio of tall plant to short plant is 3 : 1
If total plants produced are 800
No.of tall plants =(3/4) ×800 =600
No.of short plants =(1/4) ×800 =200
OR
When F1 plants were cross-pollinated with plants having tt genes, a total of 800 plants were produced. How many of these would be tall, medium height or short plants? Give the genotype of F 2 generation.
Ans.When F1 plants were cross-pollinated with plants having tt genes
The plants produced in F2 generation when F1 plants were cross-pollinated with plants having tt genes
Tt× tt → Tt,tt,Tt and tt ,it is the generation of the genotype of F2 generation
Tall plants are Tt,Tt and short plants are tt,tt
The ratio of tall plant to short plant is 2 : 2 =1:1
If total plants produced are 800
No.of tall plants =(1/2) ×800 =400
No.of short plants =(1/2) ×800 =400
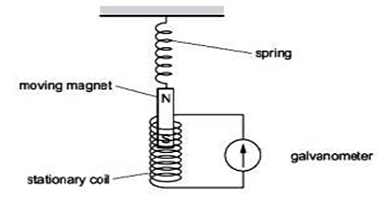
Q15.Ansari Sir was demonstrating an experiment in his class with the setup as shown in the figure below
A magnet is attached to a spring. The magnet can go in and out of the stationary coil. He lifted the Magnet and released it to make it oscillate through the coil. Based on your understanding of the phenomenon, answer the following questions.
a.What is the principle which Ansari Sir is trying to demonstrate?
Moving spring moves the magnet, as a result, it induces an electric current in the coil around it ,therefore Ansari sir is trying to demonstrate the principle of electromagnetic field induction.
b.What will be observed when the Magnet starts oscillating through the coil. Explain the reason behind this observation.
c.Consider the situation where the Magnet goes in and out of the coil. State two changes which could be made to increase the deflection in the galvanometer. OR Is there any difference in the observations in the galvanometer when the Magnet swings in and then out of the stationary coil? Justify your answer.
You can compensate us by donating any amount of money for our survival
Our Paytm No 9891436286
NCERT Solutions of Science and Maths for Class 9,10,11 and 12
NCERT Solutions of class 9 maths
NCERT Solutions of class 9 science
CBSE Class 9-Question paper of science 2020 with solutions
CBSE Class 9-Sample paper of science
CBSE Class 9-Unsolved question paper of science 2019
NCERT Solutions of class 10 maths
CBSE Class 10-Question paper of maths 2021 with solutions
CBSE Class 10-Half yearly question paper of maths 2020 with solutions
CBSE Class 10 -Question paper of maths 2020 with solutions
CBSE Class 10-Question paper of maths 2019 with solutions
NCERT solutions of class 10 science
Solutions of class 10 last year’s Science question papers
CBSE Class 10 – Question paper of science 2020 with solutions
CBSE class 10 -Latest sample paper of science
NCERT solutions of class 11 maths
| Chapter 1-Sets | Chapter 9-Sequences and Series |
| Chapter 2- Relations and functions | Chapter 10- Straight Lines |
| Chapter 3- Trigonometry | Chapter 11-Conic Sections |
| Chapter 4-Principle of mathematical induction | Chapter 12-Introduction to three Dimensional Geometry |
| Chapter 5-Complex numbers | Chapter 13- Limits and Derivatives |
| Chapter 6- Linear Inequalities | Chapter 14-Mathematical Reasoning |
| Chapter 7- Permutations and Combinations | Chapter 15- Statistics |
| Chapter 8- Binomial Theorem | Chapter 16- Probability |
CBSE Class 11-Question paper of maths 2015
CBSE Class 11 – Second unit test of maths 2021 with solutions
NCERT solutions of class 12 maths
| Chapter 1-Relations and Functions | Chapter 9-Differential Equations |
| Chapter 2-Inverse Trigonometric Functions | Chapter 10-Vector Algebra |
| Chapter 3-Matrices | Chapter 11 – Three Dimensional Geometry |
| Chapter 4-Determinants | Chapter 12-Linear Programming |
| Chapter 5- Continuity and Differentiability | Chapter 13-Probability |
| Chapter 6- Application of Derivation | CBSE Class 12- Question paper of maths 2021 with solutions |
| Chapter 7- Integrals | |
| Chapter 8-Application of Integrals |