Class 10 Science Question Paper CBSE Half Yearly Exam 2022-23 With Solutions
Check out our comprehensive resource: the Class 10 Science Question Paper CBSE Half Yearly Exam 2022-23, complete with solutions! Designed to enhance your preparation for the upcoming CBSE Board exam, this meticulously crafted paper includes a diverse array of questions likely to appear in the Science paper for the 2022-23 academic year. By delving into this practice question paper, you’ll experience a significant boost in your readiness for the Class 10 CBSE Board exam of 2022-23.
You can download PDF-Class 10 Science Question Paper CBSE Half Yearly Exam 2022-23 With Solutions
PDF-Class 10 Science Question Paper CBSE Half Yearly Exam 2022-23 With Solutions
Class 10 Science Question Paper CBSE Half Yearly Exam 2022-23 With Solutions
Understanding the significance of studying the Class 10 Science Question Paper from the CBSE Half Yearly Exam 2022-23 involves several key points:
- Comprehensive Coverage: The question paper encompasses all chapters aligned with the CBSE marking scheme, ensuring thorough preparation across the syllabus.
- Insight into Question Types: By reviewing this paper, students gain valuable insights into the types of questions likely to appear in the upcoming CBSE 2022-23 Board exam, aiding targeted preparation.
- Pathway to Excellence: Utilizing this resource effectively serves as a pathway to achieving excellent marks in the exam, as it offers a blueprint for understanding the exam pattern and expectations.
- Practice Enhancement: Regular practice with this question paper enhances proficiency in answering science questions, refining skills and familiarity with diverse question formats.
- Confidence Boost: Engaging with the question paper bolsters students’ confidence, providing them with a sense of preparedness and assurance as they approach the
Class 10 Science Question Paper CBSE Half Yearly Exam 2022-23 With Solutions
Section A
MCQ’s
Q1.The main cause of variation among organisms during sexual reproduction is:
(a) Errors in copying DNA
(b)Errors in RNA
(c)Errors in both RNA and DNA
(d)Genetic drift
Solution:(a) Errors in copying DNA
Q2.The radius of curvature of a converging mirror is 30 cm. At what distance from the mirror should an object be placed so as to obtain a virtual image?
(a) Infinity
(b)30 cm
(c)Between 15 cm and 30 cm
(d)Between 0 cm and 15 cm
Solution:
(d)Between 0 cm and 15 cm
The virual image formed by a concave mirror(converging mirror) when the object is located between focal point and the pole of the mirror.
Therefore the distance of the object should be less than its focal length (f=30/2=15 cm)
Q3. The following reaction is used for preparation of oxygen gas in laboratory:
(a) It is decomposition and endothermic
(b) It is combination reaction
(c) It is decomposition reaction and heat is released
(d) It photochemical decomposition and exothermic in nature.
Solution:(a) It is decomposition and endothermic
Q4. Which of the following is correct sequence of events of sexual reproduction in a flower?
(a) Pollination, fertilisation, seeding, embryo
(b) Seeding, embryo, fertilisation, pollination
(c) Pollination, fertilisation, embryo, seeding
(d) Embryo, seeding, pollination, fertilisation
Solution:(c) Pollination, fertilisation, embryo, seeding
Q5. What happens when dilute hydrochloric acid is added to iron fillings?
(a) Hydrogen gas and iron chloride are formed
(b) Chlorine gas and iron hydroxide are formed
(c) No reaction takes place
(d) Iron salt and water are produced
Solution:(a) Hydrogen gas and iron chloride are formed
Q6. Two conducting wires of the same material and of equal lengths and equal diameters are first connected in series and then in parallel in a circuit across the same potential difference. The ratio of heat produced in series and parallel combination would be
(a) 1:2
(b) 2:1
(c) 1:4
(d) 4:1
Solution:(c) 1:4
Heat produced in the circult = V²t/R
Let resistance of a wire is R
Net resistance of two wires when connected in series =R+R =2R
Net resistance of two wires when connected in parallel =R/2
The ratio of heat produced in series and parallel combination
= V²t/2R : V²t/R/2
=1 :4
Q7. What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) temperature increases
(ii) temperature decreases
(iii) temperature remains same
(iv) salt formation takes place
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
Solution:(d) (i) and (iv)
Q8. The refractive index of medium A is 1.5 and that of medium B is 1.33. If the speed of light in air is 3×108 m/s, what is the speed of light in mediums A and B?
(a) 2×108 m/s, 1.33×108 m/s
(b) 1.33×108 m/s, 2×108 m/s
(c) 2.25×108 m/s, 2×108 m/s
(d) 2×108 m/s, 2.25×108 m/s
Solution:Let the speed of the light in medium A is VA and in medium B is VB
Refractive index of a given medium= Velocity of the light in air/Velocity of the light in the given midium
In case of first medium
1.5 = 3×108 /VA
VA= 3×108m/s
In case of second medium
1.33= 3×108 /VB
VB= 2.25×108m/s
Q9. In the given food chain, suppose the amount of energy of fourth trophic level is 5 KJ, what will be the energy available at the producer level?
Grass → Grasshopper → Frog → Snake → Hawk
(a) 5 KJ
(B) 50 KJ
(C) 500 KJ
(D) 5000 KJ
Solution:(D) 5000 KJ
Let the energy available at the producer level is = x
The energy available at second trophic level is = 10x/100 = x/10
The energy available at third trophic level is = 10% of x/10 = x/100
The energy available at fourth trophic level is = 10% of x/100 = x/1000
Energy of fourth trophic level given is 5 KJ
x/1000 = 5 KJ
x = 5000 KJ
Q10. In a food chain, the third trophic level is always occupied by:
(a) Carnivores
(b) Herbivores
(c) Decomposers
(d) Producers
Solution:(a) Carnivores
Solution of the Science Question Paper of Class 10 half-yearly CBSE exam 2021-22 helpful for you in 2022-23 CBSE Board:see the video
Class 10 Science Question Paper CBSE Half Yearly Exam 2022-23 With Solutions
Section B
Read the assertion and reasons carefully and then mark the correct option:
(a) Both (A) and (R) are true and R is the correct explanation.
(b) Both (A) and (R) are true but R is not the correct explanation.
(c) (A) is true but (R) is false.
(d) (A) is false but (R) is true.
Q11.Assertion(A): Iron articles get rusted in moist air.
Reason(R) : Moisture and oxygen are required for rusting to form hydrated ferric oxide.
Solution:(a) Both (A) and (R) are true and R is the correct explanation.
Q12.Assertion(A): Silver articles turn black when exposed to air.
Reason(R) : Silver reacts with atmospheric oxygen.
(a) Both (A) and (R) are true and R is the correct explanation.
Q13.Assertion(A): Sodium hydroxide reacts with zinc to produce hydrogen gas.
Reason(R) : Acid reacts with active metals to produce hydrogen gas.
Solution:(b) Both (A) and (R) are true but R is not the correct explanation.
Q14.Assertion(A): Tomato contains oxalic acid,vinegar contains acetic acid
Reason(R) : Tamarind contains tartaric acid.
Solution:(b) Both (A) and (R) are true but R is not the correct explanation.
Q15.Assertion(A): In human heart, there is no mixing of oxegenated and deoxygenated blood
Reason(R) : Valves help in movement of blood in one direction.
Solution:(a) Both (A) and (R) are true and R is the correct explanation.
Q16.Assertion(A): Asexual reproduction is seen in small organisms.
Reason(R) : Budding is one type of asexual reproduction.
Solution:(b) Both (A) and (R) are true but R is not the correct explanation.
Q17.Assertion(A): The greater number of individuals are present in lower trophic level.
Reason(R) : The flow of energy is unidirectional.
Solution:(b) Both (A) and (R) are true but R is not the correct explanation.
Q18.Assertion(A): A fuse wire is always connected in parallel with the main line.
Reason(R) : If a current larger than specified value flows through the circuit,fuse wire melts.
(d) (A) is false but (R) is true.
Q19. The mirror which always forms erect and same size image is:
(a)Concave (b)Convex (c)Plane (d)Any of these
Solution:(c)Plane
Q20.When an object is kept within the focus of a concave mirror,an enlarged image is formed behind the mirror.This image is:
(a)Real (b)Inverted (c)Virtual and inverted (d)Virtual and erect
Solution: (d)Virtual and erect
Section C
Q21.Balance the following chemical equation:
NaOH +H2SO4 →Na2SO4+H2O
Solution:2NaOH +H2SO4 →Na2SO4+2H2O
Q22.A light ray travelling in air enters obliquely into kerosene oil.In what direction does the light bend and why?
Solution.A Light ray entering kerosene oil from the air will be bent towards the normal because a light ray is always bent towards the normal when it enters to a denser medium, in this case, kerosene is denser than air. The velocity of the light is slowed down when it enters to denser medium
Q23.Will current flow more easily through a thick wire or thin wire of aluminum when connected to a battery?
Solution. Current is inversely proportional to the resistance of the conductor, the resistance of the thin wire of the same metal is more than the resistance of the thick wire since resistance is inversely proportional to the area of the cross-section of the wire, therefore being lesser resistance of a thick wire the current flow more easily through it compared to thin wire of aluminum.
Q24.What would happen if mucus is not secreted by the gastric glands?
Solution. The walls of the stomach contain many gastric glands, these gastric glands secrete enzymes pepsin, mucus, and hydrochloride, mucus helps in protecting the inner lining of the stomach from the pepsin and hydrochloric acid if the mucus is not secreted by gastric glands pepsin and HCl may corrode the inner lining of the stomach which cause ulcer and acidity.
Q25.Two unequal resistances are connected in parallel.If you are not provided with any other parameters(e.g numerical values of l and R). What can be said about the voltage drop across the two resistors?
Solution. The voltage across all the unequal resistors are same if they are connected parallel to each other through a single battery or other sources of current, hence voltage drop across the two resistors is the same.
Q26.Why does the colour of copper sulphate solution change when an iron nail is dropped in it?
Solution. When iron nail is dropped in the copper sulphate solution(blue in colour),iron being more rective than copper displaces copper in the solution and forms iron sulphate (green in colour).
Fe +CuSO4(blue) →FeSO4(green)+Cu
Or
Why is the temperature of scrotal sac 2°C less than the body temprature?
Solution. The sperms are killed in the higher temperatures of the body, so it is designed to be located bellow the abdomen so for the protection of the sperms from high temperatures the temperature of the scrotal sac is 2°C less than the body temperature.
Section D
Q27.How is bleaching powder prepared? Give its chemical equation and it uses.
Solution.The chlorine gas released in the chlor alkali process is utilized in manufacturing bleaching powder. Bleaching powder is produced by the action of chlorine on dry slaked lime [Ca(OH)2].
Ca(OH)2 + Cl2 → CaOCl2 + H2O
The reaction of Cl2 on Ca(OH)2 releases bleaching powder (CaOCl2)
Use of bleaching powder: Bleaching powder is used to bleach the fabrics of cotton and linen in the textile industry,it is used to bleach wood pulp in paper factories and also used to bleach washed cloth in laundry.
Bleaching powder also used as an oxidizing agent in many chemical industries and for disinfecting drinking water to make it free of germs.
Or
A white colored powder is used by doctor for supporting fractured bones.
(i)Write the chemical name and formula of the powder.
(ii)When this white powder is mixed with water a hard solid mass is obtained .Write balanced chemical equation for the change.
Solution.
(i) The powder used by doctors for supporting fractured bones is plaster of peris whose chemical name is calcium sulfate hemihydrate.
(ii)Plaster of paris is a white powder when mixes up with water,it changes into gypsum giving hard solid mass.
Q28.A 5 cm tall object is placed perpendicular to the principal axis of a convex lens of focal length 18 cm at a distance of 12 cm from it.Use lense formula to determine the position.size and nature of image formed.
Solution.The height of the object,h = 5 cm
Focal length of the lense,f = 18 cm
The distance of the object from the lense,u=-12 cm
Applying the lense formula
1/f = 1/v – 1/u,where v is the distance of the image from the lense
1/18 = 1/v – 1/-12
1/v = 1/18 -1/12
1/v =(2-3)/36
v = -36
Position of the image is 36 cm from the lense on the same side of the object
Magnificance of the lense = v/u =h’/h,where h’ is size of the image
-36/-12 = h’/5
h’ =(-36×5)/-12 =15
Size of the image is 15 cm
Nature of the image is virtual and errect since its direction of the image is same as the direction of the object and the size of the image is positive
Q29.Bile juice does not have any digestive engyme but still plays a significant role in the process of digestion.Justify the statement.
Solution.Bile juice makes the food acidic in the small intestine coming from the stomach which is alkaline in nature since pancreatic juices can act on the acidic medium for the digestion of carbohydrates and protein, more bile breaks down larger fat globules to smaller fat globules for the action of the enzymes, the action of bile is emulsification of the fat just as the action of soap on the dirt.
Or
In birds and mammals, the left and right side of the heart are separated. Give reasons.
Solution.The separation of left and right of the heart is to prevent mixing of oxygenated and dioxygenated blood,the separation is adapted to enhance amount of oxygen supply to the blood which is required to fulfill larg energy requirement of birds and mammals in functionng of their complex organ systems.
Q30.What will be the amount of energy available to the organisms of the secondary consumer trophic level of the food chain,if the energy available to the producer level is 10000 J.
Solution. According to the 10 percent law of transferring the energy from the lower traffic level to the next trophic level,if the energy available to the producer level is 10000J then its 10 percent(i.e 1000J) will be transferred to the primary consumer(grass eating animal,second trophic level ) then 10 pecent of 1000J (i.e100J) will be transferred to secondary consumer(carnivores,third trophic level).
Name any two items which can be easily recycled but are generally thrown in the dustbin by us.
Solution. Although there are many items that are thrown to the dustbin by people and many of them can be recycled but paper and plastics are mostly thrown by people to the dustbin while of both of plastics and paper can be recycled.
Q31.Calculate the resistance of 1 cm long wire of copper of radius 1 mm. The resistivity of copper is 1.72 ×10-8 Ωm.
Solution.Applying the following formula
R =ρL/A
Resistivity of copper,ρ = 1.72 ×10-8 Ωm and length,L of the wire=1cm =10-2m
Radius,r of the copper wire= 1mm = 10-3m
Area,A of the cross section = πr² = (22/7)×(10-3)²=3.14×10-6m²
The resistance of the wire,R is
R = (1.72 ×10-8)×10-2/(3.14×10-6)
R=5.5×10-4Ω
Class 10 Science Question Paper CBSE Half Yearly Exam 2022-23 With Solutions
Section E
Q32.The figure below shows three cylindrical copper conductors along with their face areas and lengths. Compare the resistance and the resistivity of the three conductors. Justify your answer.
Solution.The relationship between resistance, length, and crossectional area of a conductor is following
R= ρL/A
Where R is the resistance,ρ is resistivity,L is length and A is area of cross-section of the conductor
In fig.(a) let the resistance of the wire is R1
R1=ρL/4A
In fig.(b) let the resistance of the wire is R2
R2=3ρL/A
In fig.(c) let the resistance of the wire is R3
R3=ρL/(3×3A)
R3=ρL/9A
Resistivity of all of these threewire is same because all the wire are of the same metal copper.
The ratio between the resistances of the (a),(b) and (c)
R1:R2:R3=ρL/4A:3ρL/A:ρL/9A
R1:R2:R3=ρL/4A:3ρL/A:ρL/9A
R1:R2:R3=9:108:4
Or
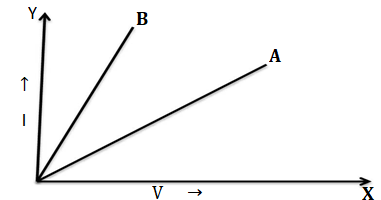
V-I graph for two wires ‘A ’ and ‘B ’ are shown in the figure.If both the wires are of the same length and are of same thickness, which of these two is made of material of higher resistivity? Give justification for your answer?
Solution.Let the resistivity of two wires ‘A ’ and ‘B ’ are ρ1 and ρ2 respectively and their resistances are R and R’
ρ1 =RA/l
ρ2 =R’A/l
ρ1 :ρ2 = R:R’
It is clear that resistivity of A and B is in the ratio of their resistances,the restance in the V-I graph is slope of the graph,the slope of B is more than the slope of A , the resistance of B is more than A therefore resistivity of B is more than A.
Q33.What is biological magnification? Why is it not desirable to suggest some methods to reduce it?
Solution. Human lies on the top level of any food chain. Several pesticides and chemicals are utilized in to protect the crop from diseases and pests. These chemicals are either washed down into the soil or into the water bodies. From the soil, these are absorbed by the plants, from the water body these are taken by fishes and other aquatic animals. These chemicals accumulated as the trophic level rises up and ultimately maximum consumption is taken place by humans,it is known as biological magnification.
It is not desirable because it can effect human health, its prolonged effect on humans may result in cancer, kidney disease, heart disease, and even birth defects.
Biological magnification can be reduced by avoiding the use of heavy metals like lead, mercury and arsenic, it can be reduced by imposing a restriction on the products which are harmful to the environment.
Or
(i) What is ozone and how is it essential for us?
(ii)What could be the consequences if non -biodegradable substances keep accumulating?
Solution.
(i)When sunlight reaches the stratosphere, the ultraviolet rays of sunlight split up oxygen molecule(O2) into its two atoms. The high reactivity of the oxygen atom forces it to combine with oxygen molecules resulting in the formation of ozone molecules.
O2 → O + O
O2+ O→ O3
Ozone(O3) is a molecule formed by three atoms of oxygen. While O2.which we normally refer to as oxygen, is essential for all aerobic forms of life.Ozone performs an essential function. It shields the surface of the earth from ultraviolet(UV) radiation from the Sun. This radiation is highly damaging to organisms, for example,it is known to cause skin cancer and cataract in human beings, UV rays also has its harmful effects on plant life and marine life.
(ii)If non -biodegradable substances plastics,metalic products like cans,water bottles,e-waste and glass etc are keep accumulating around us then its consequences would be very dangerous for environment. The accumulation of non-biodegradable substance cause the death of cattles by the ingestion of hazardous products,it will lead to biological magnification that will disorder ecological imbalance,it will lead low productivity of the crop.
Q34.1 g of copper powder is taken in a china dish and heated . What change takes place on heating? When hydrogen gas is passed over this heated substance,a visible change is seen in it. Give the chemical equations of reactions, the name, and colour of the products formed in each case.
Solution.
When 1 g of copper powder is taken in a china dish and heated, Cu is oxidized to copper oxide due to which its brown colour transforms to black
When hydrogen gas is passed over CuO then it is reduced to Cu and its colour again transforms to brown due to the formation of copper metal.
Q35.Define the term pollination. Differentiate between self-pollination and cross-pollination. What is the significance of pollination?
Solution.
Definition of Pollination: Pollination is a process of transfering pollens(male gametes) from the anther(male part) to the stigma(female part) , the pollination occurs in the flowers of the same species.
The difference between self-pollination and cross-pollination:
Self-pollination: When pollen grains are transferred from anther to stigma of the same flower then it is known as self-pollination.it occurs in bisexual flowers in in which the stigma and anther mature at the same time as an example peas, wheat, etc, for self-pollination no external medium is required.
Cross-pollination: When pollen grains are transferred from anther to stigma of the another flower then it is known as cross-pollination.it occurs in bisexual flowers in which the stigma and anther mature at the same time as an example generally it occurs in garden plants like brinjal, tomato, lady finger, etc. for cross-pollination external medium is required.
Or
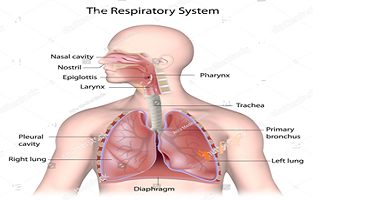
Draw a well labeled diagram of the humane respiratory system.
Section F
Q36.(i)Dry pallets of a base X when kept in open,,it absorbs moisture and turns sticky. The compound is also formed by chlor alkali process .Write chemical name and formula of X.Describe chlor alkali process with balance chemical equations.Name the type of chemical reaction which occurs when X is treated with HCl.Write the chemical equation.
(ii)While diluting the acid ,why is it recommended that acid should be added to water and not water to acid?
Solution.Dry pallets of a base X is sodium hydroxide, when it is kept in open it absorbs moisture and turns sticky,the compound NaOH is formed when electricity is passed through aqueous solution of sodium chloride.The reaction is known as chlor alkali process because the products formed are NaOH which is alkali and chlorine is shorted to chlor.
NaCl(aq) +2H2O(l) →NaOH(aq)+Cl2(g)+H2(g)
When NaOH is treated with HCl , sodium chloride(salt) and water is formed
NaOH(aq) +HCl(aq) → NaCl(aq) + H2O(l)
Or
(i)State the chemical properties on which the following uses of baking soda are based:
(a)as an antacid.
(b)as a soda acid fire extinguisher.
(c)to make bread and cake soft and spongy.
(ii)How washing soda is obtained from baking soda? Write a balanced chemical equation.
Solution.(a) Baking soda is the commercial name of sodium bicarbonate which is a base, when it reacts with HCl in the stomach it neutralizes the excess HCl, and acid reflux is relieved.
(b) Soda acid fire extinguisher contains baking soda and sulphuric acid in a different container in a cylinder when the lid of the cylinder is opened both sodium carbonate and sulphuric acid come out and reacts with each other releasing carbon dioxide and water which are responsible for extinguishing the fire.
(c) When baking soda is mixed with moisture and acid like yogurt or buttermilk a reaction takes place in which carbon dioxide is released, carbon dioxide gas expands the volume of dough of flour and creates tiny small wholes throughout it, when kept in the oven the rate of blowing carbon dioxide increases and the whole of the dough is baked up.
(ii)When baking soda (i.e sodium hydrogen carbonate) is heated, sodium carbonate, carbon dioxide, and water are formed. Sodium carbonate further reacts with water and gives the crystal of sodium hydrogen carbonate known as washing soda.
2NaHCO3 (baking soda)→ Na2 CO3 +CO2 +H2O
Na2CO3 +10H2O→ Na2CO3 .10H2O(washing soda)
Q37.(i)What is meant by food chain?”The number of trophic levels in the food chain is limited” Give reason to justify the statement.
Solution. A food chain in an ecosystem defines that one organism depends on other organisms for its survival. In a food chain energy is transferred from one organism to another organism. A food chain starts with producer organisms (Plants). All food chains are processed in the presence of bacteria on the earth.
The number of trophic levels in the food chain is limited because at each trophic level the energy is utilized for the maintenance of the organism and heat is transferred to the surroundings, as a result, a lesser amount of the energy is passed on to the next trophic level so after few levels the energy transfer is neglected.
(ii)”The flow of energy in a food chain is unidirectional”Comment.
Solution. The energy transferred in a food chain from one trophic level to the next is 10%, as an example, the first trophic level is plants which get energy from the sun, plants utilize maximum energy for their maintenance and release heat to the surroundings so 10 % of the energy is transferred to second trophic level so the transfer of energy from second trophic level to the producer is impossible as well as plants energy can’t transfer as a light energy transfer of energy can not be reverted the flow of energy in a food chain is unidirectional.
Q38.(i)Calculate the total resistance in the figure given below.
(ii)What are the advantages of parallel combination over series?
Solution.3Ω and 2Ω resistors are in series in ABD
The net resistance of (3Ω and 2Ω)in ABD = 3+2 = 5Ω
Also 3Ω and 2Ω resistors are in series in ABC
The net resistance of (3Ω and 2Ω) in ABC= 3+2 = 5Ω
Now 5Ω , 5Ω and 1Ω resistors are parallel
1/R = 1/5 +1/5 +1/1
1/R = (1+1+5)/5 =7/5
R = 5/7
Therefore the net resistance of the circuit is 5/7 Ω
(ii) The advantages of the parallel combination over series connection are
- Switching off one appliance does affect other appliance
- Overall resistance is less as compared to series connection
- Heat decay is less
- All the appliances get the full and the same voltage
- The current flows in each appliance depend only on its resistance
- In parallel combination, it is easy to connect or connect a particular appliance
Or
Figure below shows a 3Ω resistor and a 6 Ω resistor connected in parallel across a 1.5 V cell.
Calculate the current in:
(i)3Ω resistor (ii)6Ω resitor (iii)the cell
(iv)Calculate the resistance of the parallel combination.
Solution.Let the current flows through 3Ω resistor is I1and through 6 Ω resistor is I2 and current flow through the cell is I
3Ω resistor and a 6 Ω resistor are connected in parallel, their net resistance,R is
1/R = 1/3 +1/6 =3/6=1/2
R= 2Ω
The voltage,V = 1.5 V
According to the Ohm’s law
Voltage = Current×Resistance
1.5 = I1×3
I1 = 0.5 A
1.5 = I2×6
I2 = 0.25 A
The current through the cell is
1.5 = I×2
I = 0.75A
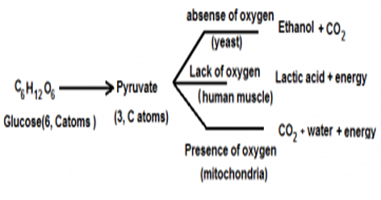
Q39.(i)What is glycolysis ? With the help of flow chart explain how glucose is broken down in different organisms to produce energy.
(ii)Differentiate between aerobic and anaerobic respiration.
Solution.
(i) Glycolysis is the primary step for cellular respiration, in this process glucose is broken down to produce energy, the process is taken place in the cytoplasm of the cell and doesn’t require oxygen because this process occurs in both aerobic and anaerobic respiration,in the process of glycolysis 2 molecules of pyruvate,ATP,NADH and water are formed.
During the cellular respiration in all organism 6 C atoms molecule of glucose is broken down into 3 C atoms molecule of pyruvate. Thereafter further pyruvate is broken in different ways in case of different organisms.
(ii)
| Aerobic respiration | Anaerobic respiration |
| It takes place in the presence of oxygen | It takes place in the absence of oxygen |
| It involves the exchange of gases between the organism and the environment | Exchange of gases between an organism and outside environment is absent |
| It occurs in cytoplasm and mitochondria | It occurs only in the cytoplasm |
| It always releases CO2 and H2O | The products formed depends on organisms |
| It yields 36 ATP’s | It yields 2 ATP’s |
Section G
Q40. Case Study
Ohms law gives the relationship between current flowing through a conductor with potential difference across it provided the physical conditions and temprature remains constant.The electric current flowing in a circuit can be measured by an ammeter.
Potential difference is measured by a voltameter connected in parallel to the battery or cell.Resistance can reduce the current in the circuit.
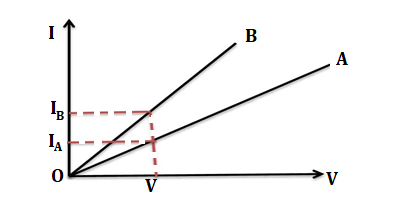
(i)Graphs between electric current and potential difference acrosss two conductors A and B are shown in the figure.Which of the following conductors has more resistance?
(a)B (b)A (c) A and B have equal resistance (d)None of these
(ii)For metallic conductors voltage vs current graph is shown at two different temperature T1 and T2.From the graph it follows.
(a)T1 = T2 (b)T1 >T2 (c)T1 < T2 (d)None of these
(iii)Seven identical lamps of resistence 220Ω each are connected to a 220V line as shown in figure.What will be the reading of ammeter?
Solution.(i) The resistance of the conductor is given as
Resistance = Voltage /Current
The currents flow on same voltage in conductor A and B are IA and IB respectively
Resistance of the conductor B = V/IB
Resistance of the conductor A = V/IA
Since IB>IA
Resistance of the conductor A >Resistance of the conductor B
(ii) Resistance of the conductor at T1 < is higher than at T2 , therefore T1 <T2
Or
Two conductors A and B of resistance 5 Ω and 10Ω respectively can be arranged in parallel and later on in series.
In each arrangement, the total voltage applied is 120 V.In which arrangement will the voltage across A and B be the same in which case will the current flowing through A and B be the same?
Solution.The net resistance of 5 Ω and 10Ω when connected in series = 5+10 =15Ω
The net resistance of 5 Ω and 10Ω when connected in parallel =(10/3)Ω
The current flow is the same in both conductors A and B when connected in series since net current will flow through the both the conductors which is
Net current through the circuit when A and B are in series =120/(5+10) =8A
In case of parallel connection current flow through A and B is as per their individual resistances
NCERT Solutions of Science and Maths for Class 9,10,11 and 12
NCERT Solutions for class 9 maths
NCERT Solutions for class 9 science
NCERT Solutions for class 10 maths
Class 10 Maths Question Paper CBSE Half Yearly Exam 2022 With Solutions
CBSE Class 10-Question paper of maths 2021 with solutions
CBSE Class 10-Half yearly question paper of maths 2020 with solutions
CBSE Class 10 -Question paper of maths 2020 with solutions
CBSE Class 10-Question paper of maths 2019 with solutions
NCERT Solutions for Class 10 Science
NCERT Solutions for class 11 maths
| Chapter 1-Sets | Chapter 9-Sequences and Series |
| Chapter 2- Relations and functions | Chapter 10- Straight Lines |
| Chapter 3- Trigonometry | Chapter 11-Conic Sections |
| Chapter 4-Principle of mathematical induction | Chapter 12-Introduction to three Dimensional Geometry |
| Chapter 5-Complex numbers | Chapter 13- Limits and Derivatives |
| Chapter 6- Linear Inequalities | Chapter 14-Mathematical Reasoning |
| Chapter 7- Permutations and Combinations | Chapter 15- Statistics |
| Chapter 8- Binomial Theorem | Chapter 16- Probability |
CBSE Class 11-Question paper of maths 2015
CBSE Class 11 – Second unit test of maths 2021 with solutions
NCERT Solutions for Class 11 Physics
chapter 3-Motion in a Straight Line
NCERT Solutions for Class 11 Chemistry
Chapter 1-Some basic concepts of chemistry
NCERT Solutions for Class 11 Biology
NCERT solutions for class 12 maths
| Chapter 1-Relations and Functions | Chapter 9-Differential Equations |
| Chapter 2-Inverse Trigonometric Functions | Chapter 10-Vector Algebra |
| Chapter 3-Matrices | Chapter 11 – Three Dimensional Geometry |
| Chapter 4-Determinants | Chapter 12-Linear Programming |
| Chapter 5- Continuity and Differentiability | Chapter 13-Probability |
| Chapter 6- Application of Derivation | CBSE Class 12- Question paper of maths 2021 with solutions |
| Chapter 7- Integrals | |
| Chapter 8-Application of Integrals |
Class 12 Solutions of Maths Latest Sample Paper Published by CBSE for 2021-22 Term 2
Class 12 Maths Important Questions-Application of Integrals
Solutions of Class 12 Maths Question Paper of Preboard -2 Exam Term-2 CBSE Board 2021-22
Solutions of class 12 maths question paper 2021 preboard exam CBSE Solution