Shapes of s,p,d and f orbitals class 11 Chemistry CBSE
An orbital is the region in which probability of finding an electron of given energy is maximum about 90% probability at any point around the nucleus is calculated using the Schrodinger wave equation.
P(finding electron) =ψ²
Where ψ² is the probability density of the electron
ψ² =0,represents the node,the point where probability of finding electron is zero and the plane passing through the node is known as nodal plane.
Quantum Numbers and Orbitals Class 11 Chemistry CBSE
Atomic radius: Periodicity in Properties of the elements
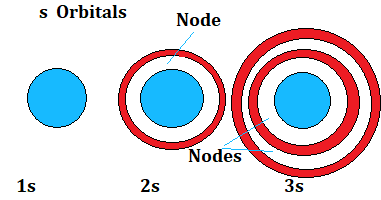
Shapes of s Orbitals:
(i) Spherical in shape
(ii) gives electron probability density at any point.
(iii)The maximum probability density of 1s electron is found near the nucleus decreases as the distance from the nucleus increases.In case of 2s electron the probability density of electron is again maximum near the nucleus and then decreases to zero and increases again and then decreases as the distance from the nucleus increases.
(iv)The intermediate region where the probability density is zero is called a nodal plane or simply node.
(v)No. of nodes (i.e spherical nodes)=n-l-1, where n is principal quantum number and l is the azimuthal quantum number.
(vi)In general any ns orbital has(n-l-1) nodes.
(vii)Electron density is equal in all directions.
Number of nodes in 1s orbital is n-l-1 = 1 -0-1 =0
Number of nodes in 2s orbital is n-l-1 = 2 -0-1 =1
Number of nodes in 3s orbital is n-l-1 = 3 -0-1 =2
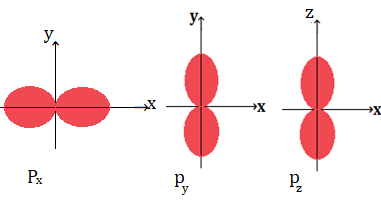
The shape of p orbital:
(i)The probability of finding the electron is maximum in two lobes on the opposite sides of the nucleus that give rise the shape of dumb bell shape for the p orbital.
(ii)A plane passing through the nucleus in which the probability of finding electrons is known as nodal plane.
(iii)p orbitals have three different orientations along x,y and z axis , these are designated as Px,py and pz
(iv)p orbitals are directional in nature.
As we know it is the magnetic quantum number which gives information of orbitals
For l =1,the value of m is -1,0 and 1,these magnetic quantum number corresponds to three orientation of p orbitals Px,py and pz
If the probability of finding electrons is along x axis then orbital shaped along x axis is known as Px
If the probability of finding electrons is along y axis then orbital shaped along y axis is known as Py
If the probability of finding electrons is along z axis then orbital shaped along z axis is known as Pz
(v)Three p orbitals belonging to a particular energy level(shell) have equal energy are called degenerate orbitals.
Shapes of d orbitals:
(i)Shape of d orbitals is double dumbled except dz²
(ii)The shape of dz² orbital is a doughnut or peanut shell.
(iii)For m=-2,-1,0,1,2,there are corresponding d orbitals of the name dxy,dyz, dzx ,dx²y²,and dz²
dxy represents the probability of finding the elctrons on x-y plane
dyz represents the probability of finding the elctrons on y-z plane
dxz represents the probability of finding the elctrons on x-z plane
dx²y² represents the probability of finding the elctrons along x and y axis
dz² represents the probability of finding the elctrons along z axis and a ring along x axis is formed which shows the probabilty of finding electrons along x -axis
You can compensate us
Paytm number 9891436286
The money collected by us will be used for the education of poor students who leaves their study because of a lack of money.
NCERT Solutions of Science and Maths for Class 9,10,11 and 12
NCERT Solutions for class 9 maths
NCERT Solutions for class 9 science
NCERT Solutions for class 10 maths
Class 10 Maths Question Paper CBSE Half Yearly Exam 2022 With Solutions
CBSE Class 10-Question paper of maths 2021 with solutions
CBSE Class 10-Half yearly question paper of maths 2020 with solutions
CBSE Class 10 -Question paper of maths 2020 with solutions
CBSE Class 10-Question paper of maths 2019 with solutions
NCERT Solutions for Class 10 Science
NCERT Solutions for class 11 maths
| Chapter 1-Sets | Chapter 9-Sequences and Series |
| Chapter 2- Relations and functions | Chapter 10- Straight Lines |
| Chapter 3- Trigonometry | Chapter 11-Conic Sections |
| Chapter 4-Principle of mathematical induction | Chapter 12-Introduction to three Dimensional Geometry |
| Chapter 5-Complex numbers | Chapter 13- Limits and Derivatives |
| Chapter 6- Linear Inequalities | Chapter 14-Mathematical Reasoning |
| Chapter 7- Permutations and Combinations | Chapter 15- Statistics |
| Chapter 8- Binomial Theorem | Chapter 16- Probability |
CBSE Class 11-Question paper of maths 2015
CBSE Class 11 – Second unit test of maths 2021 with solutions
NCERT Solutions for Class 11 Physics
chapter 3-Motion in a Straight Line
NCERT Solutions for Class 11 Chemistry
Chapter 1-Some basic concepts of chemistry
NCERT Solutions for Class 11 Biology
NCERT solutions for class 12 maths
| Chapter 1-Relations and Functions | Chapter 9-Differential Equations |
| Chapter 2-Inverse Trigonometric Functions | Chapter 10-Vector Algebra |
| Chapter 3-Matrices | Chapter 11 – Three Dimensional Geometry |
| Chapter 4-Determinants | Chapter 12-Linear Programming |
| Chapter 5- Continuity and Differentiability | Chapter 13-Probability |
| Chapter 6- Application of Derivation | CBSE Class 12- Question paper of maths 2021 with solutions |
| Chapter 7- Integrals | |
| Chapter 8-Application of Integrals |
Class 12 Solutions of Maths Latest Sample Paper Published by CBSE for 2021-22 Term 2
Class 12 Maths Important Questions-Application of Integrals
Solutions of Class 12 Maths Question Paper of Preboard -2 Exam Term-2 CBSE Board 2021-22
Solutions of class 12 maths question paper 2021 preboard exam CBSE Solution