Lewis Dot Structure Class 11 Chemistry Chapter 4 Chemical Bonding
Lewis Dot Structure Class 11 Chemistry Chapter 4 Chemical Bonding: Class 11 Chemistry Notes are very important for understanding chemistry basics. Lewis Dot Structure follows the octet rule, every atom tends to have an octet in its outermost shell, hydrogen is exceptional because its outermost shell contains 1 electron therefore it will have a tendency to obtain 2 electrons, showing the outermost electrons(valence electrons) in the representation of electronic dot structure is the Lewis dot structure, this rule can be neglected for central atom because central atom can have 8 electrons or more than 8 electrons.
Lewis Dot Structure Class 11 Chemistry Chapter 4 Chemical Bonding
The molecules or polyatomic ions contain one of the central atoms which is attached to two or more corner atoms in their electronic dot structures.
Rules for the Selecting central atom:
(i)The central atom is selected on the basis of the sequence of priority as an example the atom which is least in no/has the least electronegativity/has the largest size/has the highest atomic no.
Hydrogen and fluorine can’t be the central atom
(ii)Octet of the corner atom must be complete.
(iii) Central atom can have 8 or more electrons
(iv)Central atom tries to remain in maximum co-valency(the no. of covelents bonds formed by it),oxygen can form 1,2 ,sulphur 2,4,6, Nitrogen 3,4, Phosphorus 3,5, Carbon 4, Silicon 4, Chlorine 1,3,5,7 covalent bonds.
(v) If a positive charge is there then it will represent at central atom (Ex.H3O+,NH4+)
(vi) If the negative charge is there then it will be represented at corner atoms (NO3–,SO4-2,CO3-2)
Calculation:
Qtotal el= Valence electrons of all atoms +(any negative charge on compound if it exists) – (any positive charge on compound if it exists)
Bond Pair Electrons = 2×No. of Bonds
Lone Pair Electrons = Qtotal el– Bond Pair Electrons
Note: Either loan pair will build up or they will be utilized in making double or triple bond
Lewis Dot Structure Class 11 Chemistry Chapter 4 Chemical Bonding
Examples:
Lewis Dot Structure of Hydrogen: H2
No. of valence electrons in a single hydrogen atom = 1
Total no. of valence electrons in the molecule H2 = 2
Hence
Qtotal el= 2
H• —• H
Bond Pair Electrons =2
Lone Pair Electrons = = Qtotal el– Bond Pair Electrons = 2 – 2 =0
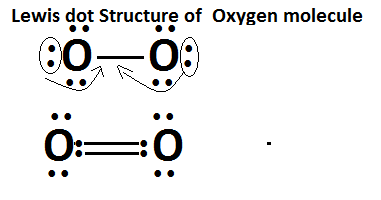
Lewis Dot Structure of Hydrogen: O2
No. of valence electrons in a single oxygen atom = 1
Total no. of valence electrons in the molecule O2 = 12
Initially placing single electron for contributing in a single covalent bond,one oxygen atom gives 2 electrons in building a single bond between oxygen atom.
Qtotal el = 6 + 6 = 12 valence electrons
Bond Pair Electrons =2
Lone Pair Electrons = = Qtotal el– Bond Pair Electrons = 12 – 2 =10
Still, the octet of both corner atoms is not completed, so for compensating it another oxygen atom will contribute its two electrons ,it is the process of bond formation between two oxygen atoms.
The charge in a molecule resonants within it, so let’s find the individual charge of each atom of the molecule which is known as a formal charge.
Formal Charge of an atom = Valence electrons of the atom – Loan pair of the atom- Bond pair electrons/2
As an example the charge of each oxygen atom in its molecule is
Formal Charge of each oxygen atom = 6 – 4 – 4/2 = 6-4-2 =0
Class 11 Physics and Chemistry Notes
Physics Notes
Forces and Newton’s First Laws of motion:Class 11 Physics Chapter 5 CBSE
Circular Motion: Angular velocity and angular displacement
Projectile Motion Class 11 CBSE Physics Chapter 4 Motion in a Plane
Addition of Vectors: CBSE Class 11 Physics Chapter 4 -Motion in a Plane
Chemistry Notes
Lattice Energy Class 11 CBSE Chemistry Chapter 4
Electronic Configuration of s,p and d orbitals
Atomic Radius Class 11 Chemistry Chapter 3 Periodicity in Properties
Why does a Rainbow look like a Bow?
NCERT Solutions of Science and Maths for Class 9,10,11 and 12
NCERT Solutions for class 9 maths
NCERT Solutions for class 9 science
NCERT Solutions for class 10 maths
Class 10 Maths Question Paper CBSE Half Yearly Exam 2022 With Solutions
CBSE Class 10-Question paper of maths 2021 with solutions
CBSE Class 10-Half yearly question paper of maths 2020 with solutions
CBSE Class 10 -Question paper of maths 2020 with solutions
CBSE Class 10-Question paper of maths 2019 with solutions
NCERT Solutions for Class 10 Science
NCERT Solutions for class 11 maths
| Chapter 1-Sets | Chapter 9-Sequences and Series |
| Chapter 2- Relations and functions | Chapter 10- Straight Lines |
| Chapter 3- Trigonometry | Chapter 11-Conic Sections |
| Chapter 4-Principle of mathematical induction | Chapter 12-Introduction to three Dimensional Geometry |
| Chapter 5-Complex numbers | Chapter 13- Limits and Derivatives |
| Chapter 6- Linear Inequalities | Chapter 14-Mathematical Reasoning |
| Chapter 7- Permutations and Combinations | Chapter 15- Statistics |
| Chapter 8- Binomial Theorem | Chapter 16- Probability |
CBSE Class 11-Question paper of maths 2015
CBSE Class 11 – Second unit test of maths 2021 with solutions
NCERT Solutions for Class 11 Physics
chapter 3-Motion in a Straight Line
NCERT Solutions for Class 11 Chemistry
Chapter 1-Some basic concepts of chemistry
NCERT Solutions for Class 11 Biology
NCERT solutions for class 12 maths
| Chapter 1-Relations and Functions | Chapter 9-Differential Equations |
| Chapter 2-Inverse Trigonometric Functions | Chapter 10-Vector Algebra |
| Chapter 3-Matrices | Chapter 11 – Three Dimensional Geometry |
| Chapter 4-Determinants | Chapter 12-Linear Programming |
| Chapter 5- Continuity and Differentiability | Chapter 13-Probability |
| Chapter 6- Application of Derivation | CBSE Class 12- Question paper of maths 2021 with solutions |
| Chapter 7- Integrals | |
| Chapter 8-Application of Integrals |
Class 12 Solutions of Maths Latest Sample Paper Published by CBSE for 2021-22 Term 2
Class 12 Maths Important Questions-Application of Integrals
Solutions of Class 12 Maths Question Paper of Preboard -2 Exam Term-2 CBSE Board 2021-22
Solutions of class 12 maths question paper 2021 preboard exam CBSE Solution