Class 10 Chemistry Practical Based Questions for CBSE 2020-21
Here class 10 chemistry practical based questions published by Future Study Point are going to help you in the preparation of science question paper CBSE board examination 2020-21. All questions are written by an expert of the subject. After studying these chemistry practical questions every student will get an idea about a few extra questions asked in science paper of board exams.
NCERT Solutions Class 10 Science from chapter 1 to 16
Science and Maths NCERT solutions for Class 9 ,10 and 11 classes
Techniques of Achieving Hundred Percent Marks in Mathematics.
Buy Class 10 physics and chemistry notes-e-book at the price of Rs 50
Class 10 Chemistry Practical Based Questions for CBSE 2020-21 Board Exam
Click for online shopping
Future Study Point.Deal: Cloths, Laptops, Computers, Mobiles, Shoes etc
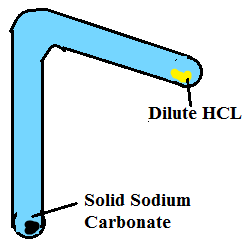
Q1. A student added dilute HCL to solid sodium carbonate and note down observation as: of
(i) No change takes place
(ii) A loud sound is produced
(iii) A brisk effervescence occurs
(iv) Solution turns blue
Correct observation is
(a) I (b) II
(c) III (d) IV
Ans.(III) A brisk effervescence occurs.
Q2. A gas produced on reaction of sodium carbonate with dilute HCL will
(i) Burn with pop sound
(ii) Be colourless and odourless
(iii) Turn lime water milky
(iv) Extinguish burning splinter if brought near it
The incorrect statement about the gas is:
(a) i (b) ii
(c) iii (d) iv
Ans.(a) i
Q3. A gas produced on reaction of zinc metal with dilute HCL
(i) Burns with a pop sound
(ii) Is colourless and odourless
(iii) Turns lime water milky
(iv) Is combustible
The incorrect statement about the gas is:
(a) I (b) II
(c) III (d) IV
Ans.(iii) Turns lime water milky
Q4.Rohit added a pinch of solid sodium carbonate to a test tube containing dilute NaOH and observes that
(i) Brisk effervescence
(ii) White ppt formed
(iii) Colorless gas produced
(iv) No change
Correct observation is:
(a) I (b) II
(c) III (d) IV
Ans.(IV) No change
Class 10 chemistry Viva Voce Questions and Answers for CBSE Board 2020-21
Q5.The metal which liberates hydrogen gas with both the acid and base(alkali)
(a) Iron (b) Zinc
(c) Copper (d) Silver
Ans.(b) Zinc
Q6.Which of the following properties are shown by dil.HCL.
(i) It turns blue litmus to red
(ii) It turns red litmus blue
(iii) It reacts with zinc to produce a colourless gas
(iv) It reacts with solid sodium carbonate to give brisk effervescence
Correct observation is:
(a) i and iii only
(b) ii and iii only
(c) i, ii and iv only
(d) i, iii and iv only
Ans.(d) i, iii and iv only
Q7.A student added dil.HCL to a test tube containing zink granules and made the following observations.
(i) The surface of zinc granules become black.
(ii) A colourless gas evolved which burn with a pop sound.
(iii) The solution remain colourless.
The correct observations are
(a) i and ii only (b) ii and iii only
(c) i and iii only (d) i, ii and iii (all)
Ans. (b) ii and iii only
Q8.When a few drops of blue litmus solutions are added to dil.HCL and aq NaOH solution taken in two different test tubes P and Q respectively. The colour changes observed will be:
(a) Blue to red in both P and Q
(b) No change in both P and Q
(c) Blue to red in P and no change in Q
(d) Blue to red in P but no change in Q
Ans.(d) Blue to red in P but no change in Q
Q9.Name of gas X liberated when contents of the test tubes shown in the figure are mixed:
(a) Carbon dioxide
(b) Hydrogen
(c) Nitrogen dioxide
(d) Sulphur dioxide
(a) Carbon dioxide
Ans.(a) Carbon dioxide
Q10.The colour of the aqueous solution of ferrous sulphate is
(a) Blue (b) Green
(c) Yellow (d) Colourless
Ans.(b) Green
Best Amazon deals
Q11.The colour of the aqueous solution of copper sulphate is
(a) Blue (b) Green
(c) White (d) Colourless
Ans.(a) Blue
Q12.The colour of the aqueous solution of aluminium sulphate is
(a) Blue (b) Green
(c) Colourless (d) White
Ans.(c) Colourless
Q13.The colour of solid aluminium sulphate salt is
(a) Blue (b) Green
(c) Colourless (d) White
Ans.(d) White
Q14.What colour change do you observe when a zink strip is dipped in ferrous sulphate solution
(a) Blue to green (b) Blue to colourless
(c) Green to colourless (d) Green to blue
Ans.(c) Green to colourless
Q15.What colour change do you observe when an iron nail is dipped in zinc sulphate solution
(a) Blue to green
(b) Blue to colourless
(c) Green to colourless
(d)No change in colour
Ans. (d)No change in colour
Q16.Which of the following salts of calcium causes permanent hardness of the water.
(a) Carbonates
(b) Hydrogen carbonates
(c) Sulphates
(d) All of these
Ans.(c)
Q17.The cause of temporary hardness of water is.
(a) Sodium hydrogen carbonate
(b) Calcium chloride
(c) Calcium hydrogen carbonate
(d) None of tbese
Ans.(c)
Q18.Washing soda is added to laundry soaps because
(a) It increases the hardness of soap.
(b) It increases the cleaning power of soap.
(c) It helps in removing any hardness present in water.
(d) None of the above.
Ans.(c)
Q19.When the soap solution is added to hard water.It forms sticky scum which is
(a) The calcium salt of long chain carboxylic acid
(b) The sodium salt of long chain carboxylic acid
(c) Hydrogen carbonates of sodium
(d) None of the above
Ans.(a)
Q20.A student takes equal quantities of distilled water in four test tubes marked as I, II, III and IV then adds a pinch of sodium chloride in test tube I, calcoum chloride in test tube II, calcium carbonate in test tube III, and potassium carbonate in test tube IV.
In which test tubes scum will be formed.
(a) II and III (b) II, III and IV
(c) II only (d) All I, II, III and IV
Ans.(a)
Q21.The cleansing capacity of any sample of soap is indicated by
(a) Its colour
(b) Time is taken by it to get dissolved in water
(c) Its foaming capacity
(d) All of these
Ans.(c)
Q22.A student arranged the set up to show that CO2 is given out during respiration, water rises.in the glass tube due to
(a) Some pressure exerted by seeds
(b)The heat released by germinating seeds
(c) Seeds are attracting the water
(d) Vacuum created due to absorption of CO2 by KOH solution
Ans.(d)
Q23.In the experiment to show the CO2 is produced during respiration, the test tube suspended in the conical flask to absorb carbon dioxide contains.
(a) Solution of dodium bicarcobate
(b) Alcohol
(c) The solution of potassium hydroxide
(d) Glycerine
Ans.(c)
Q24.Teacher advise a student to take germinating seed during respiration. The correct answer for advice.
(a) Germinating seeds create high temperature.
(b) Germinating seeds absorb O2.
(c) Germinating seeds are easy to handle
(d) Germinating seed do respiration at a faster rate to release a considerable amount of CO2.
Ans.(d)
Q25.In an experiment to show that CO2 is produced during respiration, KOH solution taken in suspended glass tube absorbs.
(a) O2 gas
(b) CO2 gas
(c) Both O2 and CO2 gas
(d) Neither O2 nor CO2 gas
Ans.(b)
Q26.To perform an experiment to show that CO2 is produced during respiration a student should use
(a) Boiled seeds (b) Dry seeds
(c) Soaked seeds (d) Germinating seeds
Ans.(d)
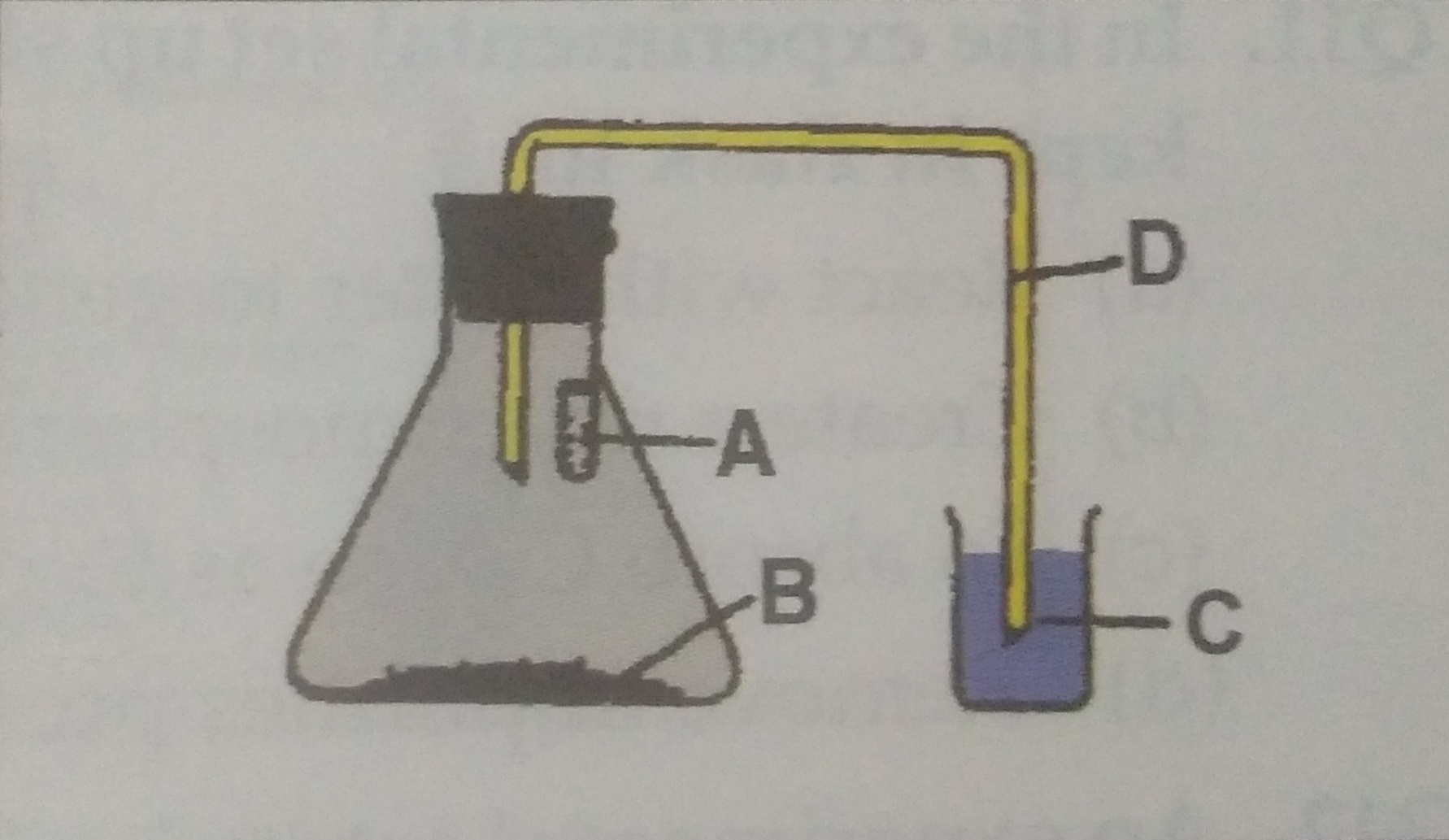
Q27. A student draws the diagram for set up of experiment to show that CO2 is produced during respiration and lebelled it as shown in fig. The correct sequence of labelling ABCD in given fig. are:
(a) Lime water, seeds, bent tube, KOH
(b) KOH, Germinating seeds, water,bent tube
(c) Water, bent tube, KOH, germinating seeds
(d) KOH, seeds, lime water, bent tube
Ans.(b)
Q28. A student was asked to set up the apparatus to show that CO2 is given out during respiration. For making the apparatus airtight he should use:
(a) Lubricant oil (b) Vaseline
(c) Cellotape (d) Fevicole
Ans. (b)
Q29.A student arranged the set up to show that CO2 is given out during respiration. But the water did not rise in the bent glass tube even after one hour of the setting of the apparatus. The correct reason may be
(a) Germinating seed do not respire
(b) Coloured water is not taken in a beaker.
(c) The round bottom flask was not used in place of conical flask
(d) Flask may not be airtight
Ans.(d)
Q30.A student followed steps given below to perform an experiment to show that CO2 is given out during respiration. The steps are not in the correct sequence
(i) Put some germinating seeds in a conical flask
(ii) Seal the mouth of the conical flask with Vaseline
(iii) Hang a small tube containing KOH solution in the flask
(iv) Fitted a cork with one hole in neck of flask.
(v) Fitted a glass tube twice at right angle in cork and other end of tube is put in water taken in beaker.
The correct sequence of steps is
(a) I, II, III, IV, V (b) V, IV, III, II, I
(c) I, III, IV, V, II (d) I, II, V, IV, III
Ans.(c) I, III, IV, V, II
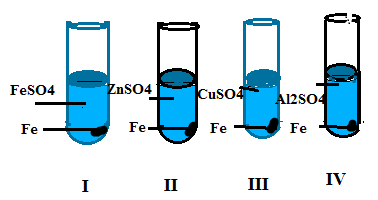
Q31. In which of the following reactions, colour change of the solution is observed
(a) I (b) II
(c) III (d) IV
Ans. (c) III
Q32. Aqueous solution CuSo4, FeSo4, ZnSo4 and Al2(So4)3, takes four test tubes, A, B, C and respectively. An aluminium strips is dipped in each test tube in which test tube colour change occurs.
(a) A and C (b) B and C
(c) C and D (d) A and B
Ans. (d) A and B
Class 10 Chemistry Important Notes
How to Balance the Chemical Reaction :Class 10 Chapter 1 NCERT
Important salts class 10 CBSE sceience notes
Why do calcium and magnesium float on the surface of the water?
Functioning of Soda-Acid Fire Extinguisher
What are Corrosion and Rancidity ?
Chemical properties of Acid and Bases-A note for grade 10 students
What is pH value and its importance in everyday life.
Type of Chemical Reactions with Complete detail
What are the physical and chemical properties of metals?
Extraction of metals as per the activity series
Trends in the property of element from left to right and up to down in the modern periodic table.
Ionic and covalent compounds and the difference between them
What is the difference between the soap and the detergent ?
Class 10 chemistry Viva Voce Questions and Answers for CBSE Board 2020-21
NCERT Solutions of Science and Maths for Class 9,10,11 and 12
NCERT Solutions for class 9 maths
NCERT Solutions for class 9 science
NCERT Solutions for class 10 maths
CBSE Class 10-Question paper of maths 2021 with solutions
CBSE Class 10-Half yearly question paper of maths 2020 with solutions
CBSE Class 10 -Question paper of maths 2020 with solutions
CBSE Class 10-Question paper of maths 2019 with solutions
NCERT Solutions for Class 10 Science
NCERT Solutions for class 11 maths
| Chapter 1-Sets | Chapter 9-Sequences and Series |
| Chapter 2- Relations and functions | Chapter 10- Straight Lines |
| Chapter 3- Trigonometry | Chapter 11-Conic Sections |
| Chapter 4-Principle of mathematical induction | Chapter 12-Introduction to three Dimensional Geometry |
| Chapter 5-Complex numbers | Chapter 13- Limits and Derivatives |
| Chapter 6- Linear Inequalities | Chapter 14-Mathematical Reasoning |
| Chapter 7- Permutations and Combinations | Chapter 15- Statistics |
| Chapter 8- Binomial Theorem | Chapter 16- Probability |
CBSE Class 11-Question paper of maths 2015
CBSE Class 11 – Second unit test of maths 2021 with solutions
NCERT Solutions for Class 11 Physics
chapter 3-Motion in a Straight Line
NCERT Solutions for Class 11 Chemistry
Chapter 1-Some basic concepts of chemistry
NCERT Solutions for Class 11 Biology
NCERT solutions for class 12 maths
| Chapter 1-Relations and Functions | Chapter 9-Differential Equations |
| Chapter 2-Inverse Trigonometric Functions | Chapter 10-Vector Algebra |
| Chapter 3-Matrices | Chapter 11 – Three Dimensional Geometry |
| Chapter 4-Determinants | Chapter 12-Linear Programming |
| Chapter 5- Continuity and Differentiability | Chapter 13-Probability |
| Chapter 6- Application of Derivation | CBSE Class 12- Question paper of maths 2021 with solutions |
| Chapter 7- Integrals | |
| Chapter 8-Application of Integrals |
Class 12 Solutions of Maths Latest Sample Paper Published by CBSE for 2021-22 Term 2
Class 12 Maths Important Questions-Application of Integrals
Solutions of Class 12 Maths Question Paper of Preboard -2 Exam Term-2 CBSE Board 2021-22
Solutions of class 12 maths question paper 2021 preboard exam CBSE Solution