CBSE Class 10 science question paper Set-3, 2020 solutions
Our purpose of presenting the solution of the 10 class CBSE science question paper 2020 board exam is to help new students of 10 class so that they could get the way of writing the solutions of science question paper in the forthcoming Class 10 CBSE board exam. The study of these solutions of science question paper of 10 class will definitely boost your preparation for the CBSE board exams 2020-21. All questions of 10 class science question paper 2020 are solved by an expert teacher of the CBSE as per the norms of CBSE board. The solution of each question is explained by a step-by-stepmethod therefore it is guaranteed that every student can understand the solutions of each question with proper understanding.
Download PDF of CBSE Class 10 science question paper 2020 SET -3 solutions
PDF Solutions class 10 CBSE science question paper 2020 board exam
Here is the complete solutions of science question paper set- 3 class X CBSE 2020, the solutions of each question is clarified by a subject expert, some of the questions are explained more than the limit with the goal that you could build up your insight, subsequently, you need not stress over whatever you have written in your worksheet or answer notebook. The fundamental target of this solution is to upgrade your knowledge base. We are likewise planning to tackle other sets of question papers of science and maths so please subscribe to our site so that you could get the information on your mobile with respect to our future posts. You can likewise write your comments for any sort of help and suggestions.
You can study our significant post on NCERT maths and science solutions of class IX, X XI and XII, articles on science and maths, post related to your carrier, blog post on the preparation of competitive exams, ebooks of science and math from class 9 to 12. After you go through the post CBSE Class 10 science question paper 2020 SET -3 solutions, tell us how did you like the solutions.
Click for most important questions of science for 2020-21
CBSE 10 class most important questions of science
NCERT solutions of class 10 science
Solutions of class 10 last years Science question papers
CBSE Class 10 – Question paper of science 2020 with solutions
CBSE class 10 -Latest Sample paper of science by CBSE
CBSE Class 10 science question paper 2020 SET -3 solutions
SECTION-A
Each Question of Section -A (Q1- Q14) is of 1 mark
Q1.Name a cyclic unsaturated carbon compound.
Ans.Benzene
Q2.State an important advantage of using alternating current(a.c) over direct current(d.c).
Ans. The A.C current of low voltage can be transported up to long distances in comparison with D.C current of low voltage due to the property of electromagnetic induction in A.C current.
Q3. Answer question numbers 3(a) to 3(d) and 4(a) to 4(d) on the basis of your understanding of the following paragraphs and the related studied concepts.
The growing size of the human population is a cause of concern for all people. The rate of birth and death in a given population will determine its size. Reproduction is the process by which organisms increase their population. The process of sexual maturation for reproduction is gradual and takes place while general body growth is still going on. Some degree of sexual maturation does not necessarily mean that the mind or body is ready for sexual acts or for having and bringing up children. Various contraceptive devices are being used by human beings to control the size of the population.
(a) List two common signs of sexual maturation in boys and girls.
(b) What is the result of reckless female foeticide?
(c) Which contraceptive method changes the hormonal balance of the body?
(d) Write two factors that determine the size of a population.
Ans.Two common signs of sexual maturation in boys and girls
In boys- (i)Growth in body hair (ii) Enlargement of testes
In girls- (i) Growth in the breast (ii) Menstruation cycle
(b) Result of reckless female foeticide is an imbalance of sex ratio of male and female in the society
(c) Birth control pills may change the hormonal balance of the body.
(d) The size of the population is determined by two factors (i) Birth rate (ii) Death rate
Set of 50 important questions of Science for Class 10-2020-21 CBSE Board.
Q4. Human body is made up of five important components, of which water is the main component. Food, as well as potable water are essential for every human being. The food is obtained from plants through agriculture. Pesticides are being used extensively for a high yield in the fields. These pesticides are absorbed by the plants from the soil along with water and minerals and from the water bodies, these pesticides are taken up by the aquatic animals and plants. As these chemicals are not biodegradable, they get accumulated progressively at each trophic level. The maximum concentration of these chemicals gets accumulated in our bodies and greatly affects the health of our mind and body.
(a) Why is the maximum concentration of pesticides found in human beings?
(b) Give one method which could be applied to reduce our intake of pesticides through food to some extent.
(c) Various steps in a food chain represent:
(a) Food web (b) Trophic level
(c) Ecosystem (d) Biomagnification
(d) With regard to various food chains operating in an ecosystem, man is a:
(a) Consumer
(b) Producer
(c) Producer and consumer
(d) Producer and decomposer
Ans.
(a) The pesticides are non-biodegradable substances which is absorbed by the plants through the soil. These Pesticides are accumulated in the water bodies due to the rainwater where it enters the body of aquatic animals, the human beings are at the top of the food chain so the maximum concentration of pesticides found in the body of the human being.
(b) Animals belong to the higher trophic level, so the concentration of pesticides is more in them than plants so increasing the habit of vegetarian food habits may reduce the concentration of pesticides in human beings.
(c) (b) Trophic level
(d) (a) Consumer
CBSE X class science updated 70 most important questions for the board exam 2020
Q5. Which one of the following is responsible for the sustenance of underground water?
(a) Loss of vegetation cover
(b) Diversion of high water demanding crops
(c) Pollution from urban wastes
(d) Afforestation
Ans. Its answer is (d) Afforestation
Explanation. Afforestation means planting the number of trees in a particular area, sustenance of underground water means to increase the water level, the tree absorbs water from the soil and prevents the land water from the process of evaporation. The roots of trees store the water and during transpiration, the clouds formed which help in rainfall in the area, it is the way how trees contribute in the water cycle and thus useful for the sustenance of groundwater.
Solutions of Class 10 Maths question paper-2019 CBSE Board
Q6.Incomplete combustion of coal and petroleum:
(A) increase air pollution (B) increases the efficiency of the machine
(C) reduces global warming (D) produces poisonous gases
The correct option is:
(a) (A) and (B) (b) (A) and (D)
(c) (B) and (C) (d) (C) and (D)
Ans.(b) (A) and (D)
Explanation. The Incomplete combustion of coal releases CO2 and Incomplete combustion of petroleum releases CO2, CO, and H2O, among them CO is considered as poisonous gas and CO2 increases pollution.
Q7. When sodium hydrogen carbonate is added to ethanoic acid a gas evolves. Consider the following statements about the gas evolved?
(A) It turns lime water milky
(B) It is evolved with a brisk effervescence
(C) It has a smell of burning sulfur
(D) It is also a by-product of respiration
The correct statements are:
(a) (A) and (B) only (b) (B) and (D) only
(c) (A), (C) and (D) (d) (A), (B) and (D)
Ans. (d) (A), (B) and (D).
Explanation.When sodium hydrogen carbonate is added to ethanoic acid following reaction occurs
CO2 turns lime water milky, it is evolved with a brisk effervescence and is also a by-product of respiration.
Q8.When a small amount of acid is added to water, the phenomena which occur are:
(A) Dilution (B) Neutralisation
(C) Formation of ions (D) Salt formation
The correct statements are:
(a) (A) and (C) (b) (B) and (D)
(c) (A) and (B) (d) (C) and (D)
Ans. (a) (A) and (C)
Explanation. The small amount of acid to water decreases the concentration of acid is known as dilution and the hydrogen atom of acid forms a bond with hydrogen and oxygen atoms of water generating hydronium ions H3O+.
Notes on the Human Digestive System
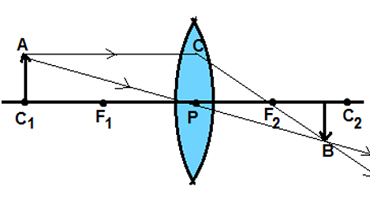
Q9. A real image is formed by the light rays after reflection or refraction when they:
(A) actually meet or intersect with each other
(B) actually converge at a point.
(C) appears to meet when they are produced in the backward direction.
(D) appear to diverge from a point.
Which of the above statements are correct ?
(a) (A) and (D) (b) (B) and (D)
(c) (A) and (B) (d) (B) and (C)
CBSE Class X Sample papers and Last years question papers of Science and Maths
Ans.(c) (A) and (B)
Explanation. As seen in the following fig. AC rays and AP rays converge and intersect at point B forming real image.
OR
Consider the following properties of virtual images:
(A) cannot be projected on the screen
(B) are formed by both concave and convex lens
(C) are always erect
(d) are always inverted
The correct properties are:
(a) (A) and (D) (b) (A) and (B)
(c) (A), (B) and (C) (d) (A), (B) and (D)
Ans.(c) (A), (B) and (C)
When the object is between Focal point and pole the image formed by the convex lens is virtual and the image formed by the concave lens is always virtual, the virtual image is always erected and can not be projected on the screen.
Q10.At the time of short circuit, the electric current in the circuit:
(a) vary continuously (b) does not change
(c) reduces substantially (d) increases heavily
Ans. (d) increases heavily
Explanation. When excess voltage exposed across two conductors then the high current flows between them as per the ohm’s law V=iR, the face wire between them heated as per the equation H = i²Rt, and come in contact with neutral wire making the path of electricity shorter due to which resistance becomes low and huge current flowed through the circuit which damages appliances.
OR
Two bulbs of 100W and 40W are connected in series. The current through the 100W bulb is 1A. The current through the 40W bulb will be:
(a) 0.4 A (b) 0.6A
(c) 0.8 A (d) 1A
Ans. (d) 1A
Explanation. In the series connection, the same current flows but the voltage across each component is according to the individual resistance of the component.
Q11. Calcium oxide reacts vigorously with water to produce slaked lime.
This reaction can be classified as
(A) Combination reaction (B) Exothermic reaction
(C) Endothermic reaction (D) Oxidation reaction
Which of the following is the correct option?
(a) (A) and (C) (B) (C) and (D)
(c) (A), (C) and (D) (d) (A) and (B)
Ans. (d) (A) and (B)
In this reaction, Cao combines with water to form calcium hydroxide, so it is a combination reaction and since heat is also released with the formation of the product hence it is an exothermic reaction also.
Click for online shopping
Future Study Point.Deal: Cloths, Laptops, Computers, Mobiles, Shoes etc
OR
When hydrogen sulfide gas is passed through a blue solution of copper sulfate, a black precipitate of copper sulfide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of a:
(a) Combination reaction (b) Displacement reaction
(c) Decomposition reaction (d) Double displacement reaction
Ans. d) Double displacement reaction
Explanation. The reaction is written as follows.
In this reaction, hydrogen and copper ions exchange each other forming copper sulfide and sulphuric acid ,so this is a double displacement reaction.
Q12. In a double displacement reaction such as the reaction between sodium sulphate solution and barium chloride solution:
(A) exchange of atoms takes place (B) exchange of ions takes place
(C) a precipitate is produced (D) an insoluble salt is produced
(a) (B) and (D) (b) (A) and (C)
(c) only (B) (d) (B), (C) and (D)
Ans.(d) (B), (C) and (D)
Explanation. The reaction between sodium sulfate solution and barium chloride solution is written as follows.
In this reaction sodium ion and barium ions substitutes each other and forms sodium chloride and insoluble white-colored salt barium sulfate is precipitated
For question numbers 13 and 14, two statements are given-one labeled Assertion (A) and the other labeled Reason (R). Select the correct answer to these questions from the codes (a), (b),(c) and (d) as given below:
(a) Both A and R are true and R is the correct explanation of the Assertion.
(b) Both A and R are true but R is not the correct explanation of the Assertion.
(c) A is true but R is false.
(d) A is false but R is true.
Q13. Assertion (A): Esterification is a process in which a sweat smelling substance is produced.
Reason(R): When esters react with sodium hydroxide an alcohol and sodium salt of carboxylic acid are obtained.
Ans. (b) Both A and R are true but R is not the correct explanation of the Assertion.
Q14. Assertion (A): A solar cooker cooks the meal due to the green house effect.
Reason(R): The plane mirror is responsible for producing the green house effect.
Ans.(b) Both A and R are true but R is not the correct explanation of the Assertion.
Explanation. Plane mirror in solar cooker works as a reflector,the glass plate inside it, is responsible for producing green house effect.
CBSE Class 10 science question paper 2020 SET -3 solutions
SECTION B
Each Question of Section -B (Q15- Q24) is of 3 marks
Q15.(a) Write the mathematical expression for Joul’s law of heating.
(b) Compute the heat generated while transferring 9600 coulombs of charge in two hours through a potential difference of 40 V.
Ans. (a) The mathematical expression for Joul’s law of heating is H = i²Rt, where H-heat energy, R- resistance in the circuit, t- time of current stayed in the circuit.
(b) The charge flow in the circuit is (Q) = 9600 C
Potential difference (V) = 40 V
The relationship between work done, voltage and the charge is given as follows
Work done is stored as heat energy
H = VQ = 40 ×9600 = 38 4000
Therefore heat generated due to the flow of current = 384000 Joule
Q16. Draw a labeled diagram to show (i) reddish appearance of the sun at the sunrise or the sunset and (ii) white appearance of the sun at noon when it is overhead
Ans. The sun appears red at the sunrise and sunset because the sun is at the largest distance from us in both of these locations, light rays have to travel larger distances, red color is being of highest wavelength scattered less and rests of the colors scattered away by the atmosphere. Only the red color reaches to our eye, it is that’s why the sun looks red.
The sun appears white at noon because light rays have to travel the shortest distance in this time, all the colors are scattered in a little quantity by the same proportion, it is that’s why the sun looks white at noon. The labeled diagrams are shown below.
Q17. (a) Draw the structure for (i) ethanol (ii) ethanoic acid
(b) Why is the conversion of ethanol to ethanoic acid considered an oxidation reaction? Write the oxidizing agent in the reaction involved.
Ans. (a)
(b) The conversion of ethanol to ethanoic acid is considered an oxidation reaction because there is the gain of the oxygen atom, as shown below, the oxidizing agents involved in the reaction is acidified potassium dichromate or Potassium permanganate.
Q18.Name the parts (a ) to (e) in the following diagram.
What is the term given to the sequence of events occurring in the diagram?
Ans. (a)→ Receptors in skin =heat/pain
(b)→ Sensory neuron
(c) → Spinal cord (CNS)
(d) → Relay neuron
(e) → Motor neuron
This is a reflex action of our body in response to the environment,as an example, if we touch a burning flame then these successive events took place. The receptor makes us to feel pain, this signal is passed by the sensory neuron to the spinal cord in turn of the relay neuron in the spinal cord communicates the motor nerve to move muscles.
OR
(a) What is tropism?
(b) How do auxins promote the growth of a tendril around a support?
Ans.(a) The plants adapt themselves according to the change in the environment, the action of plants according to the factors around it is known as tropisms. Tropisms is the growth of plants in response to stimulus as an example light, gravity, water and touch-types of tropisms are phototropism, gravitropism, hydrotropism and thigmotropism
(b) The auxins is a plant hormone, it is present in the tendril of the plants. When tendril comes in contact with anything it grows faster towards the thing due to thigmotropism it is that’s why tendril gets coiled around the support.
Solutions of Class 10 Maths question paper -2020 CBSE Board
Q19. Why is the Tyndall effect shown by colloidal particles? State four instances of observing the Tyndall effect?
Ans. The particle size of colloid is more than the particle size of the solution when the light rays fall on the colloid the light is scattered by its particle and the beam of light is visible within the solution, this phenomenon is known as Tyndall effect.
Four instances of observing the Tyndall effect are the following.
(i) In the sunbeam coming from the ventilation, the particles can be seen floating in the air.
(ii) The beam of the headlight of the car through the fog can be seen
(iii) Strip of light can be seen through the milk of glass.
(iv) The clouds look whitish in the sunshine
Q20.Define the term pollination. Differentiate between self-pollination and cross-pollination. What is the significance of pollination?
Ans. Pollination is the process of transferring pollen from anther (male organ of a flower) to the stigma (female organ of the flower). Reproduction takes place only when the pollinations occur between the species of the same plants. The pollinations occur through vector-like insects, butterflies, birds, wind, etc.
Self-pollination and cross-pollination.
| Self-pollination | Cross-pollination |
| Pollination occurs from pollens to stigma of the same flowers | Pollinations occur from pollens to the stigma of different flower |
| This process takes place on the same flower or between the two flowers of the same plant | This process takes place between two flowers of different plants |
| In this process, the next generation of plants are the homogenous progeny | In this process, the next generation of plants are the heterozygous progeny |
| Produces limited amount of pollen grains | Produces a large number of pollen grains |
| It decreases genetic variation | It increases genetic variation |
| Transfer a few numbers of pollens | Transfer a large number of pollens |
| This process is carried out even when flowers are closed | This process is carried out only when flowers are open |
Significance of pollination.
(a) The pollination increases the production of crops
(b) It is required for genetic diversity
(c) Maintains ecological balance
(d) Without this process, humans and many other organisms can not survive
How to choose your subjects after 10 or 12 pass
Q21.List the important products of the Chlor-alkali process. write one important use of each.
Ans. In the industry of the Chlor-alkali process, the brine( salt solution) is undergone through the process of electrolysis, hydrogen is collected at the negative electrode and chlorine is collected at the positive electrode, rest of the product remains sodium hydroxide.
Hydrogen. Hydrogen used as a fuel in the space rocket
Sodium hydroxide. It is used to manufacture soap
Chlorine. It is used to manufacture hydrochloric acid
Link-For free downloading games,TV shows and appes
OR
How is washing soda prepared from sodium carbonate? Give its chemical equation. State the type of salt. Name the type of hardness of water that can be removed by it?
Ans. The chemical name of washing soda is sodium carbonate decahydrate, washing soda is prepared by the process of crystalization of sodium carbonate,
Sodium carbonate is the hydrated disodium salt of carbonate acid
Q22. 1g of copper powder was taken in a china dish and heated. What change takes place on heating? When hydrogen gas is passed over this heated substance, the name and the color of the products formed in each case.
Ans. When 1g of copper powder was taken in a china dish and heated then the following reaction takes place
Cu + O2 → CuO
A black layer of copper oxide is formed on the surface of copper
When hydrogen gas is passed over CuO, black color surface changes to brown color due to the reduction of CuO to Cu(copper)
CuO + H2 → Cu +H2O
Q23. The near point of the eye of a person is 50 cm. Find the nature and power of the corrective lens required by the person to enable him to see clearly the objects placed at 25 cm from the eye.
Ans. Near point of a person is 50 cm means the person can not see the things nearer to 50 cm, the person has the defect of hypermetropia so we are needed a converging lens(convex) so that if object is placed at 25 cm the image could be formed at 50 cm which will enable him to see the object clearly.
U = –25 cm, V = –50 cm, F =?
1/F = 1/V – 1/U
1/F = 1/(–50) – 1/(–25)
1/F = –1/50 + 1/25
1/F = 1/50
F =50
The focal length of corrective lens is 50 cm
The power (P) of the lense = 1/F(in meter), F = 50/100= 0.5 m
P = 1/0.5 = 2 D
Positive sign of power signifies that the corrective lense required is a convex lense of the power 2D.
See the video for understanding Question 23.
Q24. What are homologous structures? Give an example. Is it necessary that homologous structures always have a common ancestor? Justify your answer.
Ans. The homologous structure shows the similar internal structure of two organs of two creatures but they may be used for different activities, it shows that they have a common ancestor, as an example the structure of wings bones of bats and hands of human are same but bats use wings to fly and man uses hands to grip the things, both of them are mammals shows that they have common ancestors.
The homologous structures of the species indicate that they had common ancestors, all of them are looking different because of the variation in their other parts of their body due to the environment they are living in present. According to the theory of evolution, the mammals came after the reptiles, all the mammals we are seeing today their ancestors were the same.
CBSE Class 10 science question paper 2020 SET -3 solutions
SECTION-C
Each Question of Section -C (Q25- Q30) is of 5 marks
Q25. Draw a ray diagram in each of the following cases to show the formation of image, when the object is placed:
(i) between the optical center and principal focus of a convex lens.
(ii)anywhere in front of a concave lens.
(iii)At 2F of a convex lens.
State the signs and values of magnifications in the above-mentioned cases (i) and (ii).
Ans.
magnification of lens (m)= Height of image/Height of the object = h’/h = V/U
In first case fig (i) U= F/2 and V = >2F
∴ m = V/U = >2F/(F/2) =>4
In this case, the image of the object is more than 4 times of the object length and it is virtual and erect
In the second case U ≈ 2F – F = F and V ≈ F/2
m = V/U ≈ (F/2)/F ≈ 1/2
m=h’/h =1/2
2h’= h
h’ = h/2
In this case, the image will be almost half of the size of the object and it is virtual and erect
OR
An object 4.0 cm in size is placed 25.0 cm in front of a concave mirror of focal length 15.0 cm.
(i)At what distance from the mirror should a screen be placed in order to obtain a sharp image?
(ii)Find the image of the image.
(iii)Draw a ray diagram to show the formation of the image in this case.
Ans. The focal length of the concave mirror is minus and the object is placed in front of it so U and F are in the minus sign
The size of the object (h) = 4 cm, the distance of the object(U) = –25 cm, Focal length =–15 cm
Let the screen is placed at the distance of V to obtain a sharp image
Applying the mirror formula
1/F = 1/V + 1/U
1/(–15) = 1/V + 1/(–25)
1/V = 1/25 –1/15
1/V = –2/75
V = –75/2 = –37.5
The screen should be placed at a distance of 37.5 cm in front of the mirror
(ii) Applying the magnification formula of the mirror
m = h’/h =h’/4 = –V/U = –(–37.5)/(–25) = –1.5
h’/4 = –1.5
h’ = – 6
Negative sign shows that the image of the object is inverted and its length is 6 cm
(iii)
Q26.(a)What is the law of dominance of traits? Explain with an example.
(b) Why are the traits acquired during the lifetime of an individual not inherited? Explain.
Ans.(a) ‘The law of dominance of traits’ is one of the three inheritance laws of Mendel, in the offspring of two organisms male and female of contrasting traits are crossed under the condition of monohydrate cross (only one trait of the parent is under-considered) then in first-generation one trait is dominated over other traits, as an example, Mendel’s took two pea plant of contrasting traits one is tall and other dwarf plants. In the first generation, only tall plants were generated. In this experiment, the trait of tall plants was expressed or dominated and the trait of dwarf plants was recessed or suppressed.
In the chart below since the traits are transferred in the form of two factors, pair of alleles so the trait of the tall plant is expressed as (TT) and trait of dwarf, plant is expressed as (tt)
In F1 generations all plants were heterozygous tall ( pair of alleles one is representing tallness and other is representing dwarfness but tallness is dominated).
(b) Ans. The traits acquired during the lifetime of an individual not inherited because the acquired traits are influenced by the environment in which the organism is living, these traits are not coded in the DNA of the organism so these traits can not be inherited, these traits are helpful for the organism to survive in a new environment. As an example, the traits of a great scientist who achieved a noble prize after his continuous hard work in his study can not be inherited in the next generation. The traits which can be inherited are color of hair and eye, height, skin, etc.
Click for online shopping
Future Study Point.Deal: Cloths, Laptops, Computers, Mobiles, Shoes etc
Q27.(a) A gas is released through photosynthesis. Name the gas and also state the way by which gas is evolved.
(b)What are stomata? What governs the opening and closing of stomata?
Ans (a) A gas is released through the process of photosynthesis is oxygen, the chlorophyll in the leaves of the plant absorb sunlight, subsequently, the water molecule absorbed by the roots and sent to leaves is split up into hydrogen and oxygen, the hydrogen of water molecule joins with the absorbed carbon dioxide of the atmosphere forms glucose molecule and oxygen is freed to the atmosphere. The photosynthesis reaction is as follows.
(b) The stomata are the small pores on the surface of leaves which are surrounded by guard cells, guard cells are opened in presence of the sunlight subsequently sunlight enters the cell of leaves, the light energy absorbed by chlorophyll and a chemical process occurs known as photosynthesis process. When sunlight is off the stomata are closed which prevents the water loss due to transpiration, therefore it is sunlight that governs the opening and closing of the sunlight.
OR
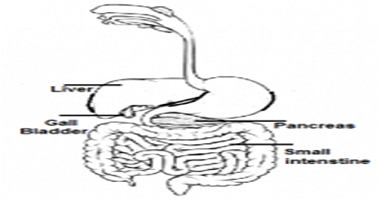
(a)Draw a diagram of the human alimentary canal and label-gall bladder, pancreas, liver and small intestine on it.
(b)Give two reasons to explain why the absorption of digested food occurs mainly in the small intestine.
Ans.(a)
Ans (b) (i) The inner surface of the small intestine has tiny fingerlike projections which are called villi, these villy increases the surface area of walls of the intestine so that the food could be absorbed
(ii) The surface of each villi is connected by the small blood capillaries through these capillaries absorbed food mixes up with the blood and distributed to each cell of the body.
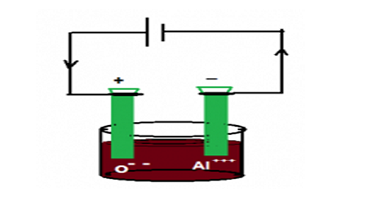
Q28.Carbon cannot reduce the oxides of sodium, magnesium, and aluminum to their respective metals. Why? wherein the reactivity series these metals are placed? How are these metals obtained from their ores? Take an example to explain the process of extraction along with chemical equations.
Ans. Carbon is less reactive than the metals like sodium, magnesium, and aluminum and is having less affinity to hold oxygen, so carbon cannot reduce the oxides of sodium, magnesium, and aluminum to their respective metals. The reactivity series in decreasing order is potassium, sodium, lithium, calcium, magnesium, aluminum, carbon, zinc, iron, copper and so on.
The way of extracting metals depends on their position in the reactivity series, if their compounds in the ore are more stable then the required energy for extracting them is more, sodium, magnesium and aluminum are more reactive substance compared to Zn, Fe, Cu, so the method of electrolysis is applied to them to extract them from their oxides.
As an example aluminum is separated from its ore bauxite (Al2O3) by the method of electrolysis as following, first of all it is melted through the furnaces and then the ore is undergone through the process of electrolysis. Aluminum is deposited at the negative electrode.
Q29.The position of certain elements in the modern periodic table are shown below.
Using the above table answer the following questions giving reasons in each case:
(i)Which element will form only covalent compounds?
(ii)Which element is a non-metal with valency 2?
(iii)Which element is a metal with valency 2?
(iv)Out of H, C, and F which has the largest atomic size?
(v) To which family does H,C, and F belong?
Ans.(i) The element which has the number of 4 electrons in its outermost orbit can form only covalent bond because it can not form its +4 and – 4 ions to form ionic bond, the element given in this table is E belongs to the group 14, the way of calculating the number of the outermost electrons 14 –10= 4, therefore The element E will form only covalent bond.
(ii) The number of electrons in the outermost orbital of group 16 elements is 16 –10= 6, which are needed 2 electrons to form anion of charge 2, so B is non-metal with valency 2
(iii) Group 2, shows that all the elements belong to this group have 2 electrons in their outermost orbit,so D is capable to donate 2 electrons hence D is the metal has a valency of 2.
(iv) H, C, and Flies on the same group, in the group, when we go down in the group the size of atom increases, so F has the highest atomic size.
(v) H, C, and F belongs to 18 groups,so the number of electrons in their outermost orbital is 18 –10= 8, which shows that these are the gases lies on the group of noble gases.
OR
Define the atomic size. Give its unit of measurement. In the modern periodic table what trend is observed in the atomic radius in a group and a period and why is it so?
Ans. The distance between the centre of atom, nucleus and outermost shell of the atom is known as atomic radii or atomic size. The unit of atomic radii is Angstrom(Å) which is equivalent to . In modern periodic table if we go down in a group the number of orbital increases , so atomic radii of the element increases and when we go from left to right the atomic size decreases because the positive charge at the centre increases which results in an increase in the force of attraction between the positively charged nucleus and the negatively charged orbitals, the orbitals shrinks up, results in the atomic radii decreases from left to right in the periodic table.
Q30.(a) Explain with the help of the pattern of magnetic field lines the distribution of the magnetic field due to current carrying a circular loop.
(b)Why is it that the magnetic field of a current-carrying coil having turned,is ‘times as large as that produced by a single turn(loop)?
Ans.(a)
Right-hand thumb rule states that if we hold a current-carrying straight wire by our hand such that the thumb directed towards the current then the fingers show the direction of the magnetic field lines, in the circular current-carrying loop the direction of current changes in four section of the loop but the direction of magnetic field changes two times, when the direction of current is from the west to east then the magnetic field around it will be anticlockwise, when the direction of electric current is towards the north, the magnetic field lines around it will be anti-clockwise, when the direction of electric current is in the direction from the east to the west, then the direction of magnetic field lines is clockwise. when the direction of current is towards the south, the direction of magnetic field lines is clockwise.
The direction of magnetic field lines is same when the direction of the current is south and the east so the resulting field lines across the circular loop is shown anticlockwise and since the direction of the field lines is same when current is in the direction of north and west so the resulting field lines are shown clockwise. The concentric circles would become larger and larger as we move towards the centre of loop where these arcs change into a straight line, the field lines originating from one of the ends of this straight line form north(N) pole and another end shows south (S) pole.
(b) The strength of the magnetic field around the circular loop is directly proportional to the current passing through it, so if there is n turns in a circular loop then the magnetic field strength will be n times of the magnetic field produced by the single circular loop, it is because the direction of the magnetic field is same in each turn, so it just adds up.
If you liked the solution in the post ‘CBSE Class 10 science question paper 2020 SET -3 solutions’ don’t forget to make a comment..
You can purchase an E-BOOK of Class 10 CBSE Science Most Important Questions With Solutions for 2020-21 board exam at Rs 30 . Click the link to buy it. Click here
E-BOOK of Class 10 CBSE Science Most Important Questions With Solutions for 2020-21 board exam
Class 10 Chemistry Important Notes
How to Balance the Chemical Reaction :Class 10 Chapter 1 NCERT
Important salts class 10 CBSE sceience notes
Why do calcium and magnesium float on the surface of the water?
Functioning of Soda-Acid Fire Extinguisher
What are Corrosion and Rancidity ?
Chemical properties of Acid and Bases-A note for grade 10 students
What is pH value and its importance in everyday life.
Type of Chemical Reactions with Complete detail
What are the physical and chemical properties of metals?
Extraction of metals as per the activity series
Trends in the property of element from left to right and up to down in the modern periodic table.
Ionic and covalent compounds and the difference between them
What is the difference between the soap and the detergent ?
Class 10 chemistry Viva Voce Questions and Answers for CBSE Board 2020-21
Class 10 Physics Important Notes
What is the difference between virtual and real images?,
Image formation by Convex and Concave Mirrors,
Difference between Convex and Concave lenses,
Why does the Sun appear reddish in the evening and morning: Complete Detail
Reflection, Refraction, Dispersion, and Scattering
Image formation by Convex and Concave Lenses,
Human Eye – Structure and functions
Myopia, Hypermetropia, and Presbyopia
Electric Current and Heating effect of Electric Current
What is a potential difference across an electric field ?
Complete detail of electrical resistance and conductance
Class X Science Important notes of chapter 12-Magnetic effect of electric current-I
Class 10 Physics Viva Voce Questions for CBSE Board 2020-21
Buy Class 10 physics and chemistry notes-e-book at the price of Rs 50
Class 10 Important Biology Notes
What is the importance of hormones?
Male and Female Reproductive System: Complete Anatomy for Grade 10 Students
The structure and anatomy of the Heart
Human digestive system structure and function
What is the difference between the homologous and analogous structure of organs
Modes of reproduction used by single organisms-Asexual reproductions
Anatomy of the Human brain-Class 10 CBSE
Ozone Layer and How it is Getting depleted.
Food chain and food web in an ecosystem
English Grammer
Antonyms and Synonyms Lists for The Preparation of CUET and other Entrance Exams
Download: Antonyms and Synonyms List
Direct and Indirect Narration rules Tenses wise and Sentences wise
Active Voice to Passive Voice Rules
Learn Tenses in English and translate Hindi sentences into English language
Download PDF-Learn Tenses in English and translate Hindi sentences into the English language
NCERT Solutions of Science and Maths for Class 9,10,11 and 12
NCERT Solutions for class 9 maths
NCERT Solutions for class 9 science
NCERT Solutions for class 10 maths
CBSE Class 10-Question paper of maths 2021 with solutions
CBSE Class 10-Half yearly question paper of maths 2020 with solutions
CBSE Class 10 -Question paper of maths 2020 with solutions
CBSE Class 10-Question paper of maths 2019 with solutions
NCERT Solutions for Class 10 Science
NCERT Solutions for class 11 maths
| Chapter 1-Sets | Chapter 9-Sequences and Series |
| Chapter 2- Relations and functions | Chapter 10- Straight Lines |
| Chapter 3- Trigonometry | Chapter 11-Conic Sections |
| Chapter 4-Principle of mathematical induction | Chapter 12-Introduction to three Dimensional Geometry |
| Chapter 5-Complex numbers | Chapter 13- Limits and Derivatives |
| Chapter 6- Linear Inequalities | Chapter 14-Mathematical Reasoning |
| Chapter 7- Permutations and Combinations | Chapter 15- Statistics |
| Chapter 8- Binomial Theorem | Chapter 16- Probability |
CBSE Class 11-Question paper of maths 2015
CBSE Class 11 – Second unit test of maths 2021 with solutions
NCERT Solutions for Class 11 Physics
chapter 3-Motion in a Straight Line
NCERT Solutions for Class 11 Chemistry
Chapter 1-Some basic concepts of chemistry
NCERT Solutions for Class 11 Biology
NCERT solutions for class 12 maths
| Chapter 1-Relations and Functions | Chapter 9-Differential Equations |
| Chapter 2-Inverse Trigonometric Functions | Chapter 10-Vector Algebra |
| Chapter 3-Matrices | Chapter 11 – Three Dimensional Geometry |
| Chapter 4-Determinants | Chapter 12-Linear Programming |
| Chapter 5- Continuity and Differentiability | Chapter 13-Probability |
| Chapter 6- Application of Derivation | CBSE Class 12- Question paper of maths 2021 with solutions |
| Chapter 7- Integrals | |
| Chapter 8-Application of Integrals |
Class 12 Solutions of Maths Latest Sample Paper Published by CBSE for 2021-22 Term 2
Class 12 Maths Important Questions-Application of Integrals
Solutions of Class 12 Maths Question Paper of Preboard -2 Exam Term-2 CBSE Board 2021-22
Solutions of class 12 maths question paper 2021 preboard exam CBSE Solution
Download e-book
Tips of developing memory power and qualifying a government entrance exams
E-BOOK of Class 10 CBSE Science Most Important Questions With Solutions for 2020-21 board exam
Class 10 Physics and Chemistry NCERT Notes
Please follow us on pintrest in.pintrest.com future study point9891